ElsevierHealthcareSeries

Expert Insights Podcast Series
2020 has been the year of the unexpected and the unconventional. The human story of the pandemic is unprecedented in the lifetime of almost every living person; the pace of scientific discovery and publication has been breathtaking; and the speed with which researchers have turned these findings to clinical use is nearly miraculous.
We have had in the Daily Rounds an introduction to vaccine development in the age of COVID, how knowledge accrued over the years has been applied, and how unconventional funding strategies have enabled vaccine development and production to proceed in parallel. We thought it would be interesting for readers to see, by contrast, the more usual course of disease discovery and vaccine development, as outlined in this presentation on HPV.
And we look forward to a time when SARS-CoV-2 vaccines are in hand, positive outcomes are established, and these Elsevier researchers can look back and apply a similar analysis to the research and development story that is unfolding before us.
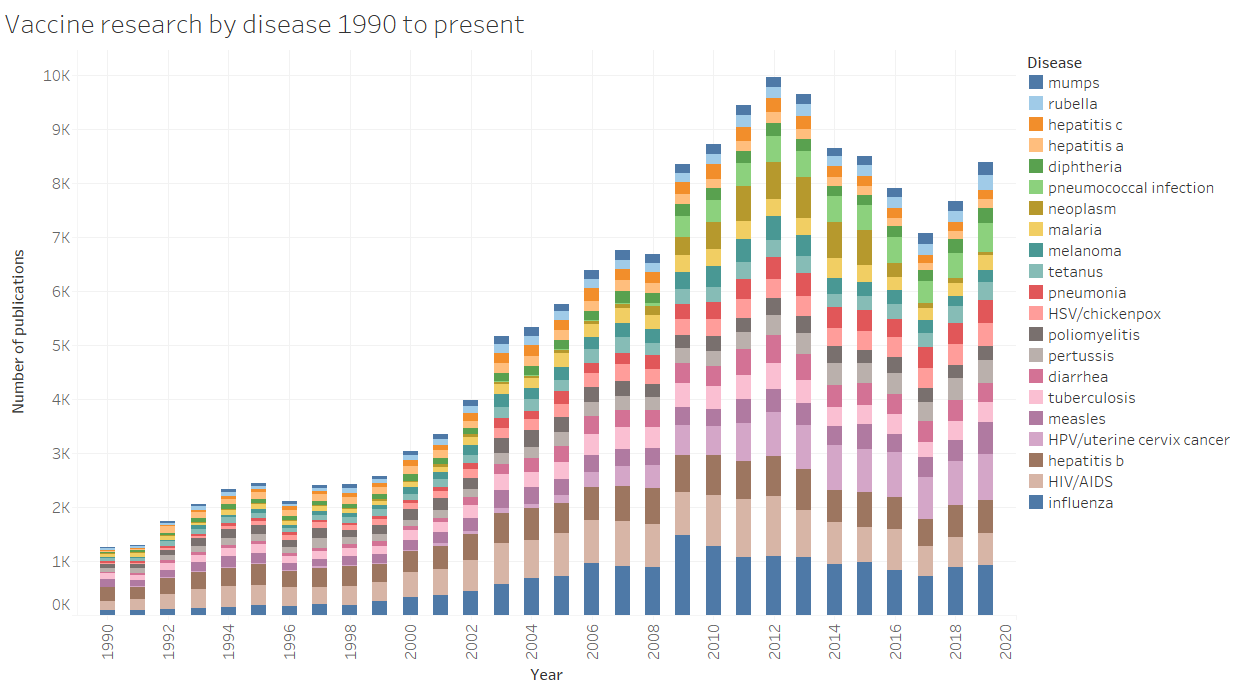
A few diseases have dominated vaccine research over the last 3 decades. These can be broadly classified as:
· Diseases for which there is a good vaccine and research tends to focus on how to improve population-level coverage and protective efficacy of the vaccine. Examples are measles, mumps, polio.
· Disease for which there has been a long struggle to make an effective vaccine. Examples are malaria, HIV/AIDS.
· Diseases for which a new vaccine has been developed within the last 3 decades. An example is HPV/cervical cancer, which first appears as a major research area in the early 2000s.
This information was shared by Bamini Jayabalasingham, PhD, Senior Analytical Product Manager, Elsevier, Analytical Services on Embase.
Although I am an Infectious Disease physician and an avid advocate of immunization, vaccine immunology and technology are not really in my wheelhouse. I find myself—like most everyone else—full of questions about the various vaccines in late stages of development and testing. What are the differences in design and mechanism, and what are the rationales behind the different approaches? How is efficacy being tested--what are the endpoints, and are they clinically relevant? Is the rapid development process shortchanging safety standards? And from an everyday practice standpoint, if several vaccines become available simultaneously, how does a practitioner decide which one to procure and administer? Or will vaccine(s) be rolled out in a massive public health initiative?
As clinical trials progress and data accrues, we will keep you informed. In the meantime, we will try to provide the background and framework with which to interpret the anticipated avalanche of information. Listen to our podcast interview with Dr. Paul Offit, the Maurice R. Hilleman Professor of Vaccinology and a Professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania, and Director of the Vaccine Education Center at the Children’s Hospital of Philadelphia. Dr. Offit was a coinventor of the RotaTeq vaccine and is a co-editor of Elsevier’s reference book Plotkin’s Vaccines. In the podcast, Dr. Offit packs a lot of information into less than 30 minutes, giving us valuable insight into the science behind the development of SARS-CoV-2 vaccines, the testing process, and potential challenges in the manufacture and widespread administration.
Expert Insights Podcast Series
Cookies são usados neste site. Para recusar ou saber mais, visite nosso conheça nosso aviso de cookies.
Copyright © 2025 Elsevier, its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.