ThisiscontentfromElsevier'sDrugInformation
Dexamethasone
Learn more about Elsevier's Drug Information today! Get the drug data and decision support you need, including TRUE Daily Updates™ including every day including weekends and holidays.
General dosing information for systemic therapy
Estimated equivalent systemic Glucocorticoid dosages. These are general approximations and may not apply to all diseases or routes of administration.[64165]
Cortisone-25 mg
Hydrocortisone-20 mg
Prednisolone-5 mg
Prednisone-5 mg
Methylprednisolone-4 mg
Triamcinolone-4 mg
Dexamethasone-0.75 mg
Betamethasone-0.75 mg
6 mg PO once daily for up to 10 days or until hospital discharge, whichever comes first is recommended by the National Institutes of Health (NIH) COVID-19 treatment guidelines for use in hospitalized patients who require supplemental oxygen, including those on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). This recommendation also applies to pregnant women, as the potential benefit of decreased maternal mortality justifies the low risk of fetal adverse effects with the short course of therapy. The NIH advises clinicians to review the patient's medical history and assess the potential risks and benefits before starting dexamethasone.[65314] The World Health Organization (WHO) strongly recommends the use of systemic corticosteroids for 7 to 10 days in patients with severe or critical COVID-19.[65876]
0.15 mg/kg/dose (Max: 6 mg/dose) PO once daily for up to 10 days, although data are limited. The National Institutes of Health (NIH) COVID-19 treatment guidelines recommend dexamethasone (with or without remdesivir) for hospitalized pediatric patients who require high-flow oxygen or noninvasive ventilation. Dexamethasone (without remdesivir) is also recommended for pediatric patients requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO). The addition of baricitinib or tocilizumab may be considered for patients who do not have rapid (e.g., within 24 hours) improvement in oxygenation after initiation of dexamethasone. Corticosteroids are not routinely recommended for pediatric patients who require only conventional oxygen, but corticosteroids can be considered in combination with remdesivir for patients with increasing oxygen needs, particularly adolescents. The use of dexamethasone for treatment of severe COVID-19 in pediatric patients who are profoundly immunocompromised has not been evaluated and may be harmful; in such cases, treatment should be considered on a case-by-case basis.[65314]
6 mg IV once daily for up to 10 days or until hospital discharge, whichever comes first is recommended by the National Institutes of Health (NIH) COVID-19 treatment guidelines for use in hospitalized patients who require supplemental oxygen, including those on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). This recommendation also applies to pregnant women, as the potential benefit of decreased maternal mortality justifies the low risk of fetal adverse effects with the short course of therapy. The NIH advises clinicians to review the patient's medical history and assess the potential risks and benefits before starting dexamethasone.[65314] The World Health Organization (WHO) strongly recommends the use of systemic corticosteroids for 7 to 10 days in patients with severe or critical COVID-19.[65876]
0.15 mg/kg/dose (Max: 6 mg/dose) IV once daily for up to 10 days, although data are limited. The National Institutes of Health (NIH) COVID-19 treatment guidelines recommend dexamethasone (with or without remdesivir) for hospitalized pediatric patients who require high-flow oxygen or noninvasive ventilation. Dexamethasone (without remdesivir) is also recommended for pediatric patients requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO). The addition of baricitinib or tocilizumab may be considered for patients who do not have rapid (e.g., within 24 hours) improvement in oxygenation after initiation of dexamethasone. Corticosteroids are not routinely recommended for pediatric patients who require only conventional oxygen, but corticosteroids can be considered in combination with remdesivir for patients with increasing oxygen needs, particularly adolescents. The use of dexamethasone for treatment of severe COVID-19 in pediatric patients who are profoundly immunocompromised has not been evaluated and may be harmful; in such cases, treatment should be considered on a case-by-case basis.[65314]
0.15 to 0.3 mg/kg/dose (Max: 6 mg/dose) PO once daily for up to 10 days is recommended as first-line immunomodulatory treatment in patients with persistent oxygen requirements due to COVID-19.[65707]
0.15 to 0.3 mg/kg/dose (Max: 6 mg/dose) IV once daily for up to 10 days is recommended as first-line immunomodulatory treatment in patients with persistent oxygen requirements due to COVID-19.[65707]
0.75 to 9 mg/day PO, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[30011] [54286] [60761]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO in 3 to 4 divided doses, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[30011] [54286] [60761] Hydrocortisone is the preferred glucocorticoid until final height is reached; however, dexamethasone may be an acceptable alternative with close monitoring.[54490]
0.5 to 9 mg/day IV or IM in 2 divided doses, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[54285] [60760]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM in 3 to 4 divided doses, initially. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[54285] [60760] Hydrocortisone is the preferred glucocorticoid until final height is reached; however, dexamethasone may be an acceptable alternative with close monitoring.[54490]
0.25 to 0.5 mg PO once daily.[54155] [68699] [71840] The FDA-approved dosage is 0.75 to 9 mg/day PO, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[30011] [54286] [60761]
0.15 to 0.375 mg/m2/dose PO once daily. Doses up to 0.71 mg/m2/day have been used. Hydrocortisone is the preferred glucocorticoid until final height is reached; however, dexamethasone may be an acceptable alternative with close monitoring.[54489] [54490] The FDA-approved dosage is 0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO in 3 to 4 divided doses, initially. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[30011] [54286] [60761]
0.25 mg PO once daily.[71840] The FDA-approved dosage is 0.75 to 9 mg/day PO, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[30011] [54286] [60761]
0.15 to 0.2 mg/m2/dose PO once daily.[54489] Hydrocortisone is the preferred glucocorticoid until final height is reached; however, dexamethasone may be an acceptable alternative with close monitoring.[54155] [54489] [54490] The FDA-approved dosage is 0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO in 3 to 4 divided doses, initially. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[30011] [54286] [60761]
0.5 mg PO every 6 hours for 48 hours. 24-hour urine collections are made for determination of 17-hydroxycorticosteroid excretion. Alternatively, 1 mg PO at 11:00 p.m. with plasma cortisol concentration measured at 8:00 a.m. the following morning.
25 to 30 mcg/kg/dose PO (Max: 2 mg/dose PO) given at 11:00 p.m. with a plasma cortisol concentration measured at 8:00 a.m. the following morning. A plasma cortisol concentration of less than 5 mcg/dL occurs in normal individuals but not those with Cushing's syndrome. Measure a dexamethasone concentration concurrently with the cortisol concentration to ensure adequacy of the dexamethasone dose.[54499]
2 mg PO every 6 hours for 48 hours. 24-hour urine collections are made for determination of 17-hydroxycorticosteroid excretion.
120 mcg/kg/dose PO (Max: 8 mg/dose PO) given at 11:00 p.m. with a plasma cortisol concentration measured at 8:00 a.m. the following morning. A decrease in the morning cortisol of 20% or more from baseline had a 97.5% sensitivity and 100% specificity in distinguishing patients with Cushing's disease from those with primary adrenal disorders in a retrospective study (n = 125, age 3 to 18 years). Measure a dexamethasone concentration concurrently with the cortisol concentration to ensure adequacy of the dexamethasone dose.[54501] Alternatively, a 2 day test consisting of 30 mcg/kg/day PO on day 1 and 120 mcg/kg/day PO on day 2, each given in 4 divided doses, has been recommended. Cortisol concentrations are suppressed in patients with pituitary Cushing's syndrome after the larger dose but not the smaller dose; cortisol concentrations are not suppressed after dexamethasone in patients with adrenocorticotropic hormone-independent Cushing syndrome.[54499]
Various dosage regimens have been used. 1 to 6 mg/kg IV or 40 mg IV every 4 to 6 hours while shock persists. Alternatively, 20 mg IV injection followed by an IV infusion of 3 mg/kg over 24 hours.[60760] Corticosteroids are given as adjunctive therapy to epinephrine.[66106] [64564]
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Adjust according to patient response.[60760] Corticosteroids are not indicated as initial treatment for anaphylaxis, but can be given as adjunctive therapy after the administration of epinephrine.[66106] [64564]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[60760] [54286] Adjust according to patient response. Corticosteroids are not indicated as initial treatment for anaphylaxis, but can be given as adjunctive therapy after the administration of epinephrine.[66106] [64564]
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Adjust according to patient response.[54286]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
4 to 8 mg IM as a single dose on day 1. Then change to oral therapy, 1.5 mg PO twice daily on days 2 and 3; then 0.75 mg PO twice daily on day 4; then 0.75 mg PO once daily on days 5 and 6, then discontinue.[54286] [54285]
5 mg IV every 6 hours for 4 doses starting the day before planned extubation. Higher doses of 10 mg every 6 hours for 4 doses did not provide additional clinical benefit.[72623] [72624] [72625]
0.5 mg/kg/dose (Max: 10 mg/dose) IV every 6 hours for 6 doses starting 6 to 12 hours before planned extubation.[54396] [54507] [64934] A prospective, randomized study (n = 153) found no significant difference in the risk of postextubation stridor, the average number of racemic epinephrine treatments, or the number of subjects requiring reintubation in subjects receiving dexamethasone compared to those receiving placebo.[54396] Another prospective, randomized study (n = 66) found that dexamethasone-treated subjects had a significantly lower rate of postextubation stridor at 10 minutes, 6 hours, and 12 hours but not 24 hours and fewer subjects requiring epinephrine or reintubation compared to those receiving placebo.[54507] A systematic review of clinical trials of dexamethasone for the prevention of postextubation stridor concluded that therapy may be beneficial in high-risk individuals, such as those with underlying airway anomalies or multiple airway manipulations.[54508]
Various regimens have been used. 0.25 mg/kg/dose IV every 8 hours for 3 doses starting approximately 4 hours before planned extubation was studied in a prospective, randomized trial in 50 premature neonates (mean gestational age, 27.7 to 28.7 weeks) who were at high risk for airway edema. The rate of postextubation stridor and reintubation was significantly lower in the dexamethasone group compared to the placebo group.[24997] A systematic review of clinical trials of dexamethasone for the prevention of extubation failure recommends therapy be reserved for use in high risk neonates, such as those with repeated or prolonged intubations, due to a lack of benefit in low risk neonates and the risk of adverse effects.[54509] Use preservative-free products for administration to neonates when possible.
10 mg IV or IM as a single dose, followed by 4 mg IV or IM every 6 hours, until symptoms subside, then reduce dosage. A response should be seen within 12 to 24 hours, and a gradual dose reduction begun after 2 to 4 days, reducing over another 5 to 7 days. Replace with oral dosage as soon as possible. For palliative maintenance therapy when oral therapy is not feasible, 2 mg IM or IV can be given 2 to 3 times per day, if needed. Use is not a substitute for neurosurgical evaluation and definitive management such as neurosurgery, etc.[60760]
For cerebral edema, 1 to 3 mg PO three times daily, can follow parenteral therapy; then, taper off over a period of 5 to 7 days.[60760] For palliative management of recurrent or inoperable brain tumors, maintenance with 2 mg PO given 2 or 3 times daily may be effective.[30011]
Initially, 1 to 1.5 mg/kg/dose IV, then 1 to 1.5 mg/kg/day IV in divided doses every 3 to 4 hours was used in conjunction with hyperventilation, control of body temperature, barbiturates, and continuous intracranial and arterial pressure monitoring in pediatric patients with severe head injury (n = 24, age 3 months to 14 years).[54512] 0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved dosage range. Adjust according to patient response. Use is not a substitute for neurosurgical evaluation and definitive management such as neurosurgery, etc.[54285] [54286]
0.02 to 0.3 mg/kg/day PO or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response. Use is not a substitute for neurosurgical evaluation and definitive management such as neurosurgery, etc.
A bolus of 8 to 10 mg dexamethasone (or equivalent) PO or IV, followed by 16 mg/day PO (usually in twice-daily to four-times-daily doses for tolerance) is a typical dose; doses are adjusted to patient condition and are either maintained or tapered over a few weeks dependent on radiation therapy cycles and/or anticipated surgery. A broad dosage range of 16 to 100 mg/day has been used depending on the presence of paraparesis, etc. Higher quality data are needed to establish the benefits vs. risks and optimal dose and duration of therapy. Experts generally agree that patients who have neurologic deficits should receive dexamethasone; many patients with MSCC require corticosteroids to help preserve neurologic function, such as ambulation.[24582] [51639]
1 to 2 mg/kg IV load followed by 0.25 to 0.5 mg/kg/dose IV every 6 hours. Max: 16 mg/dose.[64934]
NOTE: For CNS infections related to tuberculosis, see tuberculosis.
0.15 mg/kg/dose IV every 6 hours for 2 to 4 days for pneumococcal meningitis; administer the first dose 10 to 20 minutes before or concomitantly with the first dose of antimicrobial agent. Do not administer to patients who have already received antimicrobial therapy as this is unlikely to improve patient outcome.[32690]
0.15 mg/kg/dose IV every 6 hours for 2 to 4 days for H. influenzae type b; administer the first dose 10 to 20 minutes before or concomitantly with the first dose of antimicrobial agent. Do not administer to patients who have already received antimicrobial therapy as this is unlikely to improve patient outcome. Use in pneumococcal meningitis is controversial and may be considered in those older than 6 weeks of age after weighing the possible benefits and risks.[32690]
0.15 mg/kg/dose PO every 6 hours for 2 to 4 days for pneumococcal meningitis; administer the first dose 10 to 20 minutes before or concomitantly with the first dose of antimicrobial agent. Do not administer to patients who have already received antimicrobial therapy as this is unlikely to improve patient outcome.[32690]
0.15 mg/kg/dose PO every 6 hours for 2 to 4 days for H. influenzae type b; administer the first dose 10 to 20 minutes before or concomitantly with the first dose of antimicrobial agent. Do not administer to patients who have already received antimicrobial therapy as this is unlikely to improve patient outcome. Use in pneumococcal meningitis is controversial and may be considered in those older than 6 weeks of age after weighing the possible benefits and risks.[32690]
0.3 mg/kg/dose PO once daily and taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, then 3 mg PO once daily and taper by 1 mg/dose weekly for a total duration of 6 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[60760] [61094] [69585] [69587] [69589]
0.3 to 0.6 mg/kg/dose PO once daily for 4 to 6 weeks, then taper dose over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[54286] [61094] [69585] [69587] [69589] [70821]
0.3 mg/kg/dose IV or IM once daily and taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, then 3 mg IV or IM once daily and taper by 1 mg/dose weekly for a total duration of 6 weeks. Switch to oral therapy when possible. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[60760] [61094] [69585] [69587] [69589]
0.3 to 0.6 mg/kg/dose IV or IM once daily for 4 to 6 weeks, then taper dose over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[54286] [60760] [61094] [69585] [69586] [69587] [69589] [70821]
0.4 mg/kg/dose PO once daily and taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, then 4 mg PO once daily and taper by 1 mg/dose weekly for a total duration of 8 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[54286] [61094] [69585] [69587] [69589]
0.3 to 0.6 mg/kg/dose PO once daily for 4 to 6 weeks, then taper dose over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[54286] [61094] [69585] [69587] [69589] [70821]
0.4 mg/kg/dose IV or IM once daily and taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, then 4 mg IV or IM once daily and taper by 1 mg/dose weekly for a total duration of 8 weeks. Switch to oral therapy when possible. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[60760] [61094] [69585] [69587] [69589]
0.3 to 0.6 mg/kg/dose IV or IM once daily for 4 to 6 weeks, then taper dose over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[54286] [60760] [61094] [69585] [69586] [69587] [69589] [70821]
0.3 to 0.4 mg/kg/dose PO once daily for 2 to 4 weeks, then taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, and then 4 mg PO once daily and taper by 1 mg/dose weekly for a total duration of 12 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34362] [54286] [61094] [69585][69587] [69589]
0.3 to 0.4 mg/kg/dose PO once daily for 2 to 4 weeks, then taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, and then 4 mg PO once daily and taper by 1 mg/dose weekly for a total duration of 12 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34362] [54286] [61094] [69585] [69587] [69589]
0.3 to 0.6 mg/kg/dose PO once daily for 4 to 6 weeks, then taper dose over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34361] [54286] [61094] [69585] [69586] [69587] [69589] [70821]
0.3 to 0.4 mg/kg/dose IV or IM once daily for 2 to 4 weeks, then taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, and then 4 mg IV or IM once daily and taper by 1 mg/dose weekly for a total duration of 12 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34362] [60760] [61094] [69585] [69587] [69589]
0.3 to 0.4 mg/kg/dose IV or IM once daily for 2 to 4 weeks, then taper by 0.1 mg/kg/dose weekly until 0.1 mg/kg/dose, and then 4 mg IV or IM once daily and taper by 1 mg/dose weekly for a total duration of 12 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34362] [60760] [61094] [69585] [69587] [69589]
0.3 to 0.6 mg/kg/dose IV or IM once daily for 4 to 6 weeks, then taper dose over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34361] [54286] [60760] [61094] [69585] [69586] [69587] [69589] [70821]
Initially, 0.5 to 9 mg/day IV or IM, in divided doses. Adjust according to patient response. Renal transplant guidelines recommend corticosteroids for the initial treatment of acute rejection.[51730] [51731]
0.06 to 0.3 mg/kg/day or 1.2 to 10 mg/m2/day IM or IV in divided doses every 6 to 12 hours. Renal transplant guidelines recommend corticosteroids for the initial treatment of acute rejection.[51730] [51731]
Instill 3 or 4 drops (ophthalmic solution) into the aural canal 2 to 3 times per day. When a favorable response is obtained, reduce dosage gradually and eventually discontinue. If preferred, the aural canal may be packed with a gauze wick saturated with solution. Keep the wick moist with solution and remove from the ear after 12 to 24 hours. May repeat as needed at the discretion of the prescriber. There is no specific otic solution preparation; use ophthalmic solution. Used for steroid responsive inflammatory conditions of the external auditory meatus, such as allergic otitis externa, selected purulent and nonpurulent infective otitis externa when the hazard of steroid use is accepted to decrease edema and inflammation.[54348]
American Society of Clinical Oncology (ASCO) guideline-based dosage regimens are stratified according to patient risk. HIGHLY EMETOGENIC CHEMOTHERAPY: 12 mg PO or IV prior to chemotherapy, then 8 mg PO or IV on days 2 to 3 or days 2 to 4. If an NK1 receptor antagonist is not included in the anti-emetic regimen, increase to dexamethasone 20 mg PO or IV prior to chemotherapy, then 16 mg PO or IV on days 2 to 3 or days 2 to 4. MODERATELY EMETOGENIC CHEMOTHERAPY: 8 mg PO or IV prior to chemotherapy, then 8 mg PO or IV on days 2 and 3. LOW EMOTOGENIC RISK CHEMOTHERAPY: 8 mg PO or IV as a single dose prior to chemotherapy.[63197] (NOTE: Other regimens have been used historically during chemotherapy - e.g., 10 to 20 mg IV before administration of chemotherapy, with additional, lower doses given for 24 to 72 hours, as needed).
10 to 14 mg/m2/dose IV is usually used prior to chemotherapy. A 5-HT3 antagonist is usually given along with dexamethasone for highly-emetogenic chemotherapy. An example regimen: dexamethasone 10 mg/m2/dose IV once daily, along with ondansetron. Some patients receive repeat dexamethasone every 12 hours, either IV or PO, but optimal regimens for repeat dosing are not established. For chemotherapy that is less emetogenic, doses as low as 6 mg/m2/dose PO have been given. The optimal dose of steroids for chemotherapy-induced nausea/vomiting (CINV) in children is not determined, and there are safety considerations.[49435] [54434]
0.75 to 9 mg/day PO in 2 to 4 divided doses. Adjust dose according to response.[30011]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO in 3 to 4 divided doses, initially. Adjust dose according to response.[54286]
0.5 to 9 mg/day IV or IM in 2 to 4 divided doses. Adjust dose according to response.[60760]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM in 3 to 4 divided doses. Adjust dose according to response.[54286] [60760]
8 to 16 mg or 0.1 to 0.3 mg/kg/dose IV or IM once daily, then taper dose over 7 to 10 days.[68070] [68102]
0.1 to 0.3 mg/kg/dose IV or IM once daily, then taper dose over 7 to 10 days.[68070] [68102]
100 mg or 1 to 1.5 mg/kg/dose IV or IM once daily for 3 days.[68070] [68100] [68101]
1 to 1.5 mg/kg/dose IV or IM once daily for 3 days.[68070] [68100] [68101]
2 to 6 mg by intralesional injection; may repeat dose every 3 to 5 days to every 2 to 3 weeks. Dosage dependent upon degree of inflammation, size, disease state, and location of affected area. Usually employed when condition to be treated is limited to 1 or 2 sites.[60760]
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Adjust according to patient response.[30011]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Adjust maintenance dosage according to patient response.[60760]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
Dosage ranges from 2 to 4 mg for large joints and 0.8 to 1 mg for small joints. Injection into intervertebral joints should not be attempted at any time and hip joint injection cannot be recommended as an office procedure. Intrasynovial should be employed only when affected areas are limited to 1 or 2 sites. May repeat from once every 3 to 5 days to once every 2 to 3 weeks.[60760]
The 4 mg/mL injection strength may be used for intralesional and soft tissue administration. Doses range from 0.2 mg to 4 mg injected as a single dose at the appropriate site. For soft tissue and bursal injections a dose of 2 to 4 mg is recommended. Ganglia require a dose of 1 to 2 mg. A dose of 0.4 to 1 mg is used for injection into tendon sheaths. Usually employed when condition to be treated is limited to 1 or 2 sites. Dosage dependent upon degree of inflammation, size, disease state, and location of affected area. Repeat doses may be given from once every 3 to 5 days to once every 2 to 3 weeks.[60760]
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. For many conditions, the dosing of corticosteroids is highly variable. Adjust according to patient response. In an open study of 10 patients with ITP, pulse dosing produced a sustained improvement in platelet count with a total daily dose of 40 mg/day PO for 4 consecutive days out of each 28 day cycle for 6 consecutive cycles.[24390]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
Initially, 0.5 to 9 mg/day IV or IM, given in 2 to 4 divided doses. For many conditions, the dosing of corticosteroids is highly variable. Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Dosage of corticosteroids can be highly variable, depending on patient condition. Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response. Administer dexamethasone IV or IM initially for the treatment of severe respiratory conditions or those compromising the airway.
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Dosage of corticosteroids can be highly variable, depending on patient condition. Adjust according to patient response.[54557]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response. Administer dexamethasone IV or IM initially for the treatment of severe respiratory conditions or those compromising the airway.
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Adjust according to patient response.
0.06 to 0.3 mg/kg/day or 1.2 to 10 mg/m2/day IV or IM, in divided doses every 6 to 12 hours.
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Adjust according to patient response.[54286] Use of dexamethasone for longer than 2 days may increase the potential for metabolic side effects. Use parenteral dexamethasone dosage for severe respiratory conditions or those compromising the airway.[69016]
0.6 mg/kg/dose PO as a single dose or once daily for 2 days. Max: 16 mg/dose.[54531] [54533] [59736] [59737] [64934] [69016] Administer dexamethasone IV or IM initially for the treatment of severe respiratory conditions or those compromising the airway. Single or 2-day regimens of dexamethasone have shown similar efficacy, less vomiting, and improved compliance when compared to a 5-day course of oral prednisone or prednisolone.[54531] [54533] [59736] [59737] [69016] Use of dexamethasone for longer than 2 days may increase the potential for metabolic side effects.[69016] Of note, 0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved initial dosage range for dexamethasone; however, this is significantly lower than the range used in clinical practice.[54286]
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Adjust according to patient response.[54285] Use of dexamethasone for longer than 2 days may increase the potential for metabolic side effects.[69016]
0.6 mg/kg/dose IV or IM as a single dose or once daily for 2 days. Max: 16 mg/dose.[54357] [59738] [64934] [69016] Single-dose regimens ranging from 0.3 to 1.7 mg/kg/dose have been reported. Max: 36 mg/dose.[59736] In a study of young children with moderate exacerbations, a single day regimen of parenteral dexamethasone resulted in similar efficacy as a 5-day course of oral prednisolone.[59738] Use of dexamethasone for longer than 2 days may increase the potential for metabolic side effects.[69016] Of note, 0.5 to 9 mg per day IV or IM is the FDA-approved initial dosage range depending on the condition being treated; however, higher doses are sometimes used in clinical practice.[54285] [54286]
0.15 to 0.6 mg/kg/dose (Usual Max: 16 mg/dose) PO as a single dose.[54351] [54542] [54543] [59648] [64934]
0.15 to 0.6 mg/kg/dose (Usual Max: 16 mg/dose) IV or IM as a single dose.[54542] [54544] [64934]
0.4 mg/kg/dose PO once daily or every other day as needed for symptom control has been used.[33558] Long-term systemic corticosteroid treatment is only recommended as last resort in patients with severe asthma and only after specialist consultation.[69016] Consider add-on low dose oral corticosteroids (7.5 mg/day or less of prednisone equivalent) only for those with poor symptom control and/or frequent exacerbation despite good inhaler technique and treatment adherence. Guidelines suggest dexamethasone syrup as an alternative in pediatric patients unable to tolerate liquid prednisone or prednisolone.[33558] However, other guidelines do not recommend routine use except for asthma exacerbations due to the risk for metabolic side effects when dexamethasone is used beyond 2 days.[69016]
6 mg IM every 12 hours for 4 doses between 24 and 34 weeks gestation with risk for preterm delivery within 7 days. Use may also be considered starting at 22 weeks gestation if neonatal resuscitation is planned and after appropriate counseling. If labor is impending and further doses are unlikely, the first dose of dexamethasone should still be given because treatment with corticosteroids for less than 24 hours is still associated with a significant reduction in neonatal morbidity/mortality. However, no additional benefit has been demonstrated for courses of antenatal steroids with shorter dosage intervals than those recommended, often referred to as accelerated dosing, even when delivery is imminent. A repeat or rescue course of corticosteroids may be considered when less than 34 weeks gestation, with risk of preterm delivery within the next 7 days, and whose prior course of antenatal corticosteroids was administered more than 14 days previously. Rescue course corticosteroids could be provided as early as 7 days from the prior dose if indicated by clinical situation.[64435] [69147] [69183] [69184]
6 mg IM every 12 hours for 4 doses between 24 and 34 weeks gestation with risk for preterm delivery within 7 days. Use may also be considered starting at 22 weeks gestation if neonatal resuscitation is planned and after appropriate counseling. If labor is impending and further doses are unlikely, the first dose of dexamethasone should still be given because treatment with corticosteroids for less than 24 hours is still associated with a significant reduction in neonatal morbidity/mortality. However, no additional benefit has been demonstrated for courses of antenatal steroids with shorter dosage intervals than those recommended, often referred to as accelerated dosing, even when delivery is imminent. A repeat or rescue course of corticosteroids may be considered when less than 34 weeks gestation, with risk of preterm delivery within the next 7 days, and whose prior course of antenatal corticosteroids was administered more than 14 days previously. Rescue course corticosteroids could be provided as early as 7 days from the prior dose if indicated by clinical situation.[64435] [69147] [69183] [69184]
Numerous dosing schedules have been studied. The Dexamethasone: A Randomized Trial (DART) study (n = 70, median gestational age 25 weeks) used the following tapering dose schedule over 10 days: 0.075 mg/kg/dose IV twice daily for 3 days, followed by 0.05 mg/kg/dose IV twice daily for 3 days, followed by 0.025 mg/kg/dose IV twice daily for 2 days, followed by 0.01 mg/kg/dose IV twice daily for 2 days. This dosing regimen facilitated extubation by day 10 but did not significantly improve mortality or oxygen dependence at 36 weeks; follow-up at 2 years of age did not indicate any significant adverse neurodevelopmental outcomes in neonates treated with dexamethasone.[54555] [54556] Use is somewhat controversial, and most experts suggest using low doses and careful patient selection. The American Academy of Pediatrics (AAP) recommends against the use of high-dose dexamethasone (more than 0.5 mg/kg/day) due to the risk of short- and long-term adverse effects, including neurodevelopmental effects.[54338] Late corticosteroid therapy (initiated after 7 days of age) may be preferred over early therapy (initiated at less than 7 days of age). Late therapy may reduce neonatal mortality without significantly increasing potential adverse long-term neurodevelopmental outcomes.[64673] [64674] [70386] [70387]
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. For many conditions, the dosing of corticosteroids is highly variable. Adjust to patient response.[30011]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Adjust according to patient response until urine is protein-free, then slowly taper as indicated. Some patients may require long-term dosing.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
Dosages vary depending upon the chemotherapy protocol. Common doses include 1.5 to 6 mg/m2/day PO for 8 to 21 days or 8 mg PO every 8 hours for 10 days.
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Dosing can be quite variable, depending on the patient's condition. Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
initial, 0.75 to 9 mg orally daily; dose is dependent on the disease being treated and should be individualized based on patient response. Maintenance therapy may be given; use the lowest dose that produces an adequate clinical response. Taper dexamethasone gradually in patients on long-term therapy; do not abruptly stop therapy in these patients.[60761]
initial, 0.02 to 0.3 mg/kg (0.6 mg to 9 mg/m2) orally daily in 3 or 4 divided doses. Dose is dependent on the disease being treated and should be individualized based on patient response. Maintenance therapy may be given; use the lowest dose that produces an adequate clinical response. Taper dexamethasone gradually in patients on long-term therapy; do not abruptly stop therapy in these patients.[60761]
initial, 0.5 to 9 mg IV daily; dose is dependent on the disease being treated and should be individualized based on patient response. Maintenance therapy may be given; use the lowest dose that produces an adequate clinical response. Taper dexamethasone gradually in patients receiving IV therapy for more than a few days; do not abruptly stop therapy in these patients.[60760]
40 mg orally daily on days 1, 2, 3, and 4 in combination with gemcitabine 1,000 mg/m2 IV on days 1 and 8 and cisplatin 75 mg/m2 IV on day 1 (GDP regimen) every 21 days for 2 cycles was evaluated in a randomized, phase III trial (NCIC-CTG LY.12 trial). In patients with CD20-positive lymphoma, rituximab 375 mg/m2 IV was added on day 1 of each treatment cycle (R-GDP regimen). Patients in the trial could receive a third cycle of therapy if they did not achieve a complete or partial response after the second cycle. Patients with CD20-positive lymphoma who received an autologous stem-cell transplant (ASCT) were randomized to receive either rituximab 375 mg/m2 IV every 2 months for 6 cycles or observation starting 28 days post ASCT.[60756]
40 mg orally or IV on days 1, 2, 3, and 4 as part of the DHAP regimen with cisplatin 100 mg/m2 as a continuous IV infusion over 24 hours on day 1 and cytarabine 2 grams/m2 IV over 3 hours every 12 hours for 2 doses on day 2 in combination with ofatumumab 1,000 mg IV on days 1 and 8 of cycle 1 then ofatumumab 1,000 mg IV on day 1 of cycles 2 and 3 was evaluated in a randomized, phase III trial (n = 445; the ORCHARRD trial). Cycles were repeated every 21 days for a total of 3 cycles of therapy. Premedication with acetaminophen, diphenhydramine, and an IV glucocorticoid was administered prior to each ofatumumab infusion. If dexamethasone from the DHAP chemotherapy was dosed on the same day as ofatumumab, then the glucocorticoid premedication was omitted and substituted with the 40-mg dose of dexamethasone. Granulocyte colony-stimulating factor use was recommended as follows: filgrastim 5 micrograms (mcg)/kg on days 6 to 13 or pegfilgrastim 6 mg on day 6 on cycles of therapy with no stem-cell mobilization and filgrastim 5 to 10 mcg/kg on days 6 to 13 on cycles of therapy that were followed by stem-cell mobilization. Central nervous system prophylaxis using intrathecal therapy was permitted. Supportive care during treatment consisted of irradiated blood products, oral antibiotics, and antifungal prophylaxis as clinically indicated.[61715]
6 to 10 mg/m2/day PO for 14 days as part of induction, consolidation, or intensification combination regimens.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range [54286]; however, doses may vary according to the specific protocol used.
Initially, 0.5 to 9 mg IV or IM daily; dose is dependent on the disease being treated and should be individualized based on patient response. Maintenance therapy may be given; use the lowest dose that produces an adequate response. Taper dexamethasone gradually in patients receiving parenteral therapy for more than a few days; do not abruptly stop treatment.[60760]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range [54285] [54286]; however, doses may vary according to the specific protocol used.
NOTE: Dexamethasone has been designated an orphan drug by the FDA for the treatment of multiple myeloma.
40 mg orally on days 1, 8, 15, and 22; administer in combination with lenalidomide (25 mg orally daily for 21 days followed by 7 days off treatment). Continue 28-day treatment cycles until disease progression in patients who are ineligible for an autologous stem-cell transplantation (ASCT); hematopoietic stem-cell mobilization should occur within four 28-day treatment cycles in patients who are eligible for an ASCT.[65868] [58806]
20 mg orally on days 1, 8, 15, and 22; administer in combination with lenalidomide (25 mg orally daily for 21 days followed by 7 days off treatment). Continue 28-day treatment cycles until disease progression.[65868] [58806]
40 mg orally daily on days 1 to 4, 9 to 12, and 17 to 20 every 28 days for the first 4 cycles of therapy, and then 40 mg orally daily on days 1 to 4 every 28 days starting with cycle 5. Given in combination with lenalidomide (25 mg orally daily on days 1 to 21 of each cycle). Continue or modify dosing based on clinical and laboratory findings.[65868] [58806]
40 mg orally daily on days 1 to 4, days 9 to 12, and days 17 to 20 of every 28-day treatment cycle plus thalidomide 200 mg orally daily (given at bedtime and at least 1 hour after the evening meal).[65868] [49713]
40 mg PO or IV on days 1, 8, 15, and 22 in combination with lenalidomide (25 mg orally daily for 21 days) and carfilzomib as specified in the protocol.[65868] Treatment cycles are repeated every 28 days until disease progression or unacceptable toxicity; maximum of 18 cycles for carfilzomib only. CYCLE 1: carfilzomib 20 mg/m2 IV over 10 minutes on days 1 and 2; if tolerated, increase to a target dose of 27 mg/m2 IV over 10 minutes on days 8, 9, 15, and 16. CYCLES 2 to 12: carfilzomib 27 mg/m2 IV over 10 minutes on days 1, 2, 8, 9, 15, and 16. CYCLES 13 to 18: carfilzomib 27 mg/m2 IV over 10 minutes on days 1, 2, 15, and 16. Dose carfilzomib at a maximum body surface area (BSA) of 2.2 m2; dose adjustment is not necessary for patients with a weight change of 20% or less. Give dexamethasone 30 minutes to 4 hours prior to the carfilzomib (on carfilzomib dosing days only). Give hydration with both oral fluids and IV fluids prior to each carfilzomib dose in cycle 1. Additional IV hydration may be given after the carfilzomib infusion in cycle 1. Oral and/or IV hydration may be continued as needed in subsequent cycles; adjust hydration to individual patient needs. Thromboprophylaxis is recommended. Consider giving an antiviral agent and an antacid medication.[51306] In a prespecified interim analysis of a multinational, randomized, open-label, phase 3 trial (n = 792; the ASPIRE trial), the median progression-free survival time (primary endpoint) was significantly increased with carfilzomib plus lenalidomide/dexamethasone (26.3 months) compared with lenalidomide/dexamethasone alone (17.6 months; hazard ratio (HR) = 0.69; 95% CI, 0.57 to 0.83; p = 0.0001) in patients with relapsed multiple myeloma who had received 1 to 3 prior therapies (age range, 31 to 91 years; median of 2 prior therapies). In this study, some patients had previously received bortezomib (65.8%) and/or lenalidomide (19.8%). The median overall survival (OS) time had not been reached in either study arm at the time of the interim analysis (median follow-up: carfilzomib arm, 32.3 months; lenalidomide/dexamethasone alone, 31.5 months). The estimated 24-month OS rates were 73.3% and 65% in the carfilzomib/lenalidomide/dexamethasone and lenalidomide/dexamethasone arms, respectively (HR = 0.79; 95% CI, 0.63 to 0.99; p = 0.04); prespecified criteria for stopping the study due to OS benefit was not met and this study is ongoing.[60044]
20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 during cycles 1 to 8, then dexamethasone 20 mg orally on days 1, 2, 8, and 9 during cycles 9 to 16.[65868] Administer in combination with bortezomib (1.3 mg/m2 IV bolus over 3 to 5 seconds on days 1, 4, 8, and 11 in cycles 1 to 8) then bortezomib (1.3 mg/m2 on days 1 and 8 in cycles 9 to 16) and panobinostat (20 mg orally on days 1, 3, 5, 8, 10, and 12). Continue every 21-day treatment cycles for up to 8 cycles; consider giving up to an additional 8 cycles (maximum of 16 treatment cycles) in patients who experience clinical benefit without unresolved severe or medically significant toxicity.[58821] Treatment with panobinostat, bortezomib, and dexamethasone (n = 387; median therapy duration of 5 months) was compared with placebo, bortezomib, and dexamethasone (n = 381; median therapy duration of 6.1 months) in patients with relapsed or relapsed and refractory multiple myeloma who had received 1 to 3 prior therapies in a multinational, randomized, phase 3 trial (the PANORAMA1 trial). The median patient age was 63 years (range, 56 to 69 years), about 51% of patients had received 1 prior therapy, and approximately 57% of patients had previously received a stem-cell transplantation. Patients with primary refractory or bortezomib-refractory disease were ineligible for this study. At a median follow-up time of 6.47 months (interquartile range, 1.81 to 13.47 months), the median progression-free survival time (primary endpoint) was significantly improved in the panobinostat arm (11.99 months) compared with the placebo arm (8.08 months; hazard ratio (HR) = 0.63; 95% CI, 0.52 to 0.76; p less than 0.0001). The overall survival (OS) time was not significantly improved in the panobinostat arm (33.64 months vs. 30.39 months; HR = 0.87; 95% CI, 0.69 to 1.1); however, OS data are not mature. Crossover from the placebo arm to the panobinostat arm was not permitted.[58822]
20 mg orally or IV on days 1, 2, 4, 5, 8, 9, 11, and 12 (or 20 mg orally/IV once weekly in patients with a body-mass index less than 18.5, poorly controlled diabetes mellitus, or a prior intolerance to glucocorticoid therapy) repeated every 3 weeks for 8 cycles in combination with daratumumab and bortezomib.[65868] The bortezomib dosage is 1.3 mg/m2 as a subcutaneous injection or IV infusion on days 1, 4, 8, and 11 repeated every 3 weeks for 8 cycles. The daratumumab dosage is 16 mg/kg (actual body weight) IV weekly on weeks 1 to 9 (9 doses), 16 mg/kg IV every 3 weeks on weeks 10 to 24 (5 doses), and then 16 mg/kg IV every 4 weeks starting on week 25 until disease progression. Administer standard pre-and post-infusion medications with daratumumab infusions. Give dexamethasone prior to the daratumumab infusion when these drugs are scheduled on the same day.[61207] [60311]
20 mg orally or IV once weekly repeated every 3 weeks for 8 cycles in combination with daratumumab and bortezomib.[65868] The bortezomib dosage is 1.3 mg/m2 as a subcutaneous injection or IV infusion on days 1, 4, 8, and 11 repeated every 3 weeks for 8 cycles. The daratumumab dosage is 16 mg/kg (actual body weight) IV weekly on weeks 1 to 9 (9 doses), 16 mg/kg IV every 3 weeks on weeks 10 to 24 (5 doses), and then 16 mg/kg IV every 4 weeks starting on week 25 until disease progression. Administer standard pre-and post-infusion medications with daratumumab infusions. Give dexamethasone prior to the daratumumab infusion when these drugs are scheduled on the same day.[61207] [60311]
40 mg orally or IV once weekly (or 20 mg orally or IV once weekly for patients with a body-mass index less than 18.5) in combination with lenalidomide and daratumumab until disease progression or unacceptable toxicity.[65868] The lenalidomide dosage is 25 mg orally daily on days 1 to 21 repeated every 28 days in patients with creatinine clearance (CrCl) greater than 60 mL/min and 10 mg orally daily on days 1 to 21 repeated every 28 days in patients with a CrCl of 30 to 60 mL/min. The daratumumab dosage is 16 mg/kg (actual body weight) IV weekly on weeks 1 to 8 (8 doses), 16 mg/kg IV every other week on weeks 9 to 24 (8 doses), and then 16 mg/kg IV every 4 weeks starting on week 25 until disease progression. Administer standard pre-and post-infusion medications with daratumumab infusions. In patients receiving full dose dexamethasone, administer as 20 mg IV prior to the daratumumab infusion and then 20 mg PO the next day when these drugs are scheduled on the same week; patients receiving a 20 mg/week dexamethasone dose should receive the entire dose administered prior to the daratumumab infusion.[61407] [60311]
20 mg orally or IV once weekly in combination with lenalidomide and daratumumab until disease progression or unacceptable toxicity.[65868] The lenalidomide dosage is 25 mg orally daily on days 1 to 21 repeated every 28 days in patients with creatinine clearance (CrCl) greater than 60 mL/min and 10 mg orally daily on days 1 to 21 repeated every 28 days in patients with a CrCl of 30 to 60 mL/min. The daratumumab dosage is 16 mg/kg (actual body weight) IV weekly on weeks 1 to 8 (8 doses), 16 mg/kg IV every 2 weeks on weeks 9 to 24 (8 doses), and then 16 mg/kg IV every 4 weeks starting on week 25 until disease progression. Administer standard pre-and post-infusion medications with daratumumab infusions. Give dexamethasone prior to the daratumumab infusion when these drugs are scheduled on the same week.[61407] [60311]
40 mg orally or IV once weekly (or 20 mg orally or IV once weekly for patients with a body-mass index less than 18.5) in combination with lenalidomide and daratumumab until disease progression or unacceptable toxicity.[65868] Give dexamethasone IV prior to the first infusion; oral administration may be considered thereafter. Give the treatment dexamethasone dose as the daratumumab premedication steroid when these drugs are scheduled on the same day. Consider giving a low-dose oral corticosteroid (equivalent to methylprednisolone 20 mg or less) on the day after every infusion. The lenalidomide dosage is 25 mg orally daily on days 1 to 21 repeated every 28 days in patients with creatinine clearance (CrCl) greater than 50 mL/min and 10 mg orally daily on days 1 to 21 repeated every 28 days in patients with a CrCl of 30 to 50 mL/min. The daratumumab dosage is 16 mg/kg (actual body weight) IV weekly on weeks 1 to 8 (8 doses), 16 mg/kg IV every 2 weeks on weeks 9 to 24 (8 doses), and then 16 mg/kg IV every 4 weeks starting on week 25 until disease progression. Administer standard pre-and post-infusion medications with daratumumab infusions.[60311] In the MAIA trial (median follow-up, 56.2 months), the median progression-free survival (time not reached vs. 34.4 months; hazard ratio (HR) = 0.53; 95% CI, 0.43 to 0.66, p less than 0.0001) and overall survival (time not reached in either arm; HR = 0.68; 95% CI, 0.53 to 0.86) times were significantly improved in the daratumumab plus lenalidomide and dexamethasone arm compared with the lenalidomide and dexamethasone arm in patients (median age, 73 years; range, 45 to 90 years) with newly diagnosed multiple myeloma who were ineligible for a stem-cell transplant.[67219]
20 mg orally or IV once weekly in combination with lenalidomide and daratumumab until disease progression or unacceptable toxicity.[65868] Give dexamethasone IV prior to the first infusion; oral administration may be considered thereafter. Give the treatment dexamethasone dose as the daratumumab premedication steroid when these drugs are scheduled on the same day. Consider giving a low-dose oral corticosteroid (equivalent to methylprednisolone 20 mg or less) on the day after every infusion. The lenalidomide dosage is 25 mg orally daily on days 1 to 21 repeated every 28 days in patients with creatinine clearance (CrCl) greater than 50 mL/min and 10 mg orally daily on days 1 to 21 repeated every 28 days in patients with a CrCl of 30 to 50 mL/min. The daratumumab dosage is 16 mg/kg (actual body weight) IV weekly on weeks 1 to 8 (8 doses), 16 mg/kg IV every 2 weeks on weeks 9 to 24 (8 doses), and then 16 mg/kg IV every 4 weeks starting on week 25 until disease progression. Administer standard pre-and post-infusion medications with daratumumab infusions. Give dexamethasone prior to the daratumumab infusion when these drugs are scheduled on the same week.[60311] In the MAIA trial (median follow-up, 56.2 months), the median progression-free survival (time not reached vs. 34.4 months; hazard ratio (HR) = 0.53; 95% CI, 0.43 to 0.66, p less than 0.0001) and overall survival (time not reached in either arm; HR = 0.68; 95% CI, 0.53 to 0.86) times were significantly improved in the daratumumab plus lenalidomide and dexamethasone arm compared with the lenalidomide and dexamethasone arm in patients (median age, 73 years; range, 45 to 90 years) with newly diagnosed multiple myeloma who were ineligible for a stem-cell transplant.[67219]
40 mg IV/PO (or 20 mg PO/IV in patients with a body-mass index less than 18.5) once weekly plus lenalidomide 25 mg PO daily on days 1 to 21 repeated every 28 days in combination with 1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), 1,800 mg daratumumab and 30,000 units hyaluronidase every other week on weeks 9 to 24 (8 doses), and then 1,800 mg daratumumab and 30,000 units hyaluronidase every 4 weeks starting on week 25 until disease progression was evaluated in a single-arm cohort (n = 65) of a multicohort, open-label trial (the PLEIADES trial). The overall response rate was 91% in patients with relapsed or refractory multiple myeloma who received daratumumab/hyaluronidase, lenalidomide, and dexamethasone.[65868] [65366]
20 mg PO/IV once weekly plus lenalidomide 25 mg PO daily on days 1 to 21 repeated every 28 days in combination with 1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), 1,800 mg daratumumab and 30,000 units hyaluronidase every other week on weeks 9 to 24 (8 doses), and then 1,800 mg daratumumab and 30,000 units hyaluronidase every 4 weeks starting on week 25 until disease progression was evaluated in a single-arm cohort (n = 65) of a multicohort, open-label trial (the PLEIADES trial). The overall response rate was 91% in patients with relapsed or refractory multiple myeloma who received daratumumab/hyaluronidase, lenalidomide, and dexamethasone.[65868] [65366]
40 mg orally or IV once weekly (or 20 mg IV/PO once weekly for patients with a body-mass index less than 18.5) in combination with pomalidomide (4 mg orally daily on days 1 to 21 repeated every 28 days) and daratumumab (16 mg/kg of actual body weight IV weekly on weeks 1 to 8 (8 doses), 16 mg/kg IV every other week on weeks 9 to 24 (8 doses), and then 16 mg/kg IV every 4 weeks starting on week 25) until disease progression was evaluated in a nonrandomized, phase 1b trial (n = 103; EQUULEUS trial). Administer standard pre-and post-infusion medications with daratumumab infusions. In patients receiving full dose dexamethasone, administer as 20 mg IV prior to the daratumumab infusion and then 20 mg orally the next day when these drugs are scheduled on the same week; patients receiving a 20 mg/week dexamethasone dose should receive the entire dose administered prior to the daratumumab infusion.[65868] [60311]
20 mg orally or IV once weekly in combination with pomalidomide (4 mg orally daily on days 1 to 21 repeated every 28 days) and daratumumab (16 mg/kg of actual body weight IV weekly on weeks 1 to 8 (8 doses), 16 mg/kg IV every other week on weeks 9 to 24 (8 doses), and then 16 mg/kg IV every 4 weeks starting on week 25) until disease progression was evaluated in a nonrandomized, phase 1b trial (n = 103; EQUULEUS trial). Administer standard pre-and post-infusion medications with daratumumab infusions. Give dexamethasone prior to the daratumumab infusion when these drugs are scheduled on the same week. [65868] [60311]
28 mg orally (taken 3 to 24 hours prior to elotuzumab) on days 1, 8, 15, and 22 on cycles 1 and 2 and on days 1 and 15 of subsequent cycles in combination with lenalidomide 25 mg orally daily on days 1 through 21 and elotuzumab 10 mg/kg IV once weekly on cycles 1 and 2 (on days 1, 8, 15, and 22), then 10 mg/kg IV every 2 weeks (on days 1 and 15) thereafter. Give dexamethasone 40 mg orally on days 8 and 22 of cycles 3 and beyond. Repeat treatment cycles every 28 days until disease progression.[65868] Administer the following premedications 45 to 90 minutes prior to each elotuzumab infusion: acetaminophen 650 to 1,000 mg PO, diphenhydramine 25 to 50 mg PO or IV (or equivalent), ranitidine 50 mg IV or 150 mg PO (or equivalent), and dexamethasone 8 mg IV.[60354] At a median follow-up time of 24.5 months, the median progression-free survival time was significantly improved with elotuzumab plus lenalidomide and dexamethasone (median duration of therapy, 17 months) compared with lenalidomide and dexamethasone alone (19.4 months vs. 14.9 months; hazard ratio (HR) = 0.7; 95% CI, 0.57 to 0.85; p less than 0.001) in patients with relapsed and/or refractory multiple myeloma in a planned interim analysis of a multicenter, randomized, open-label, phase 3 trial (n = 646; the ELOQUENT-2 trial). In this study, patients had received a median of 2 prior therapies (range, 1 to 4 therapies); 35% of patients had refractory disease to the last therapy and 54% of patients had previously received an autologous stem cell transplantation.[60353] The overall survival time was improved in the elotuzumab-containing arm (48.3 months vs. 39.6 months; HR = 0.82; 95% CI, 0.68 to 1) at the final analysis (minimum follow-up time of 70.6 months). In subgroup analyses, the median OS times were significantly improved in elotuzumab-treated patients who had received 2 or 3 prior therapies (51 months vs. 33.6 months; HR = 0.71; 95% CI, 0.54 to 0.92), were refractory to their most recent therapy (40.4 months vs. 25.9 months; HR = 0.67; 95% CI, 0.49 to 0.91), or were less than 65 years of age (63.5 months vs. 47.7 months; HR = 0.7; 95% CI, 0.52 to 0.96).[65918]
28 mg orally (at 3 to 24 hours prior to elotuzumab) on days 1, 8, 15, and 22 on cycles 1 and 2 and on day 1 of subsequent cycles in combination with elotuzumab 10 mg/kg IV once weekly on cycles 1 and 2 (on days 1, 8, 15, and 22) followed by 20 mg/kg IV every 4 weeks (on day 1) starting on cycle 3 and pomalidomide 4 mg orally daily on days 1 through 21. Additionally, give dexamethasone 40 mg (at 3 to 24 hours prior to elotuzumab) on days 8, 15, and 22 of cycles 3 and beyond. Repeat treatment cycles every 28 days until disease progression.[65868] Administer the following premedications 45 to 90 minutes prior to each elotuzumab infusion: acetaminophen 650 to 1,000 mg orally, diphenhydramine 25 to 50 mg orally or IV (or equivalent), ranitidine 50 mg IV or 150 mg orally (or equivalent), and dexamethasone 8 mg IV. At a minimum follow-up time of 9.1 months, the median investigator-assessed progression-free survival time was significantly improved with elotuzumab plus pomalidomide and dexamethasone (median number of treatment cycles, 9) compared with pomalidomide and dexamethasone alone (10.25 months vs. 4.67 months; hazard ratio (HR) = 0.54; 95% CI, 0.34 to 0.86; p = 0.0078) in patients with relapsed or refractory multiple myeloma in a randomized, phase 2 trial (n = 117; the ELOQUENT-3 trial). In this study, patients had received a median of 3 prior therapies; 70% of patients had refractory disease after both lenalidomide and a proteasome inhibitor and 55% of patients had previously received an autologous stem cell transplantation.[60354]
8 mg orally (at 3 to 24 hours prior to elotuzumab) on days 1, 8, 15, and 22 on cycles 1 and 2 and on day 1 of subsequent cycles in combination with elotuzumab 10 mg/kg IV once weekly on cycles 1 and 2 (on days 1, 8, 15, and 22) followed by 20 mg/kg IV every 4 weeks (on day 1) starting on cycle 3 and pomalidomide 4 mg orally daily on days 1 through 21. Additionally, give dexamethasone 20 mg orally (at 3 to 24 hours prior to elotuzumab) on days 8, 15, and 22 of cycles 3 and beyond. Repeat treatment cycles every 28 days until disease progression.[65868] Administer the following premedications 45 to 90 minutes prior to each elotuzumab infusion: acetaminophen 650 to 1,000 mg orally, diphenhydramine 25 to 50 mg orally or IV (or equivalent), ranitidine 50 mg IV or 150 mg orally (or equivalent), and dexamethasone 8 mg IV. At a minimum follow-up time of 9.1 months, the median investigator-assessed progression-free survival time was significantly improved with elotuzumab plus pomalidomide and dexamethasone (median number of treatment cycles, 9) compared with pomalidomide and dexamethasone alone (10.25 months vs. 4.67 months; hazard ratio (HR) = 0.54; 95% CI, 0.34 to 0.86; p = 0.0078) in patients with relapsed or refractory multiple myeloma in a randomized, phase 2 trial (n = 117; the ELOQUENT-3 trial). In this study, patients had received a median of 3 prior therapies; 70% of patients had refractory disease after both lenalidomide and a proteasome inhibitor and 55% of patients had previously received an autologous stem cell transplantation.[60354]
20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 repeated every 21 days for 8 cycles (SWOG S0777 trial); 20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 repeated every 21 days for 3 cycles prior to stem-cell transplantation (SCT) followed by 10 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 repeated every 21 days for 2 cycles after SCT (IFM 2009 trial); and 20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 repeated every 21 days for 4 cycles, 10 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 on cycles 5 to 9, and then 10 mg orally on days 1, 2, 8, and 9 on cycles 9 to 12 (ENDURANCE trial) in combination with bortezomib and lenalidomide (VRd regimen) have been evaluated in 3 randomized, phase 3 trials. Maintenance therapy consisted of lenalidomide and dexamethasone or lenalidomide only.[61788] [65843] [65899]
40 mg orally daily on days 1 to 4 during all cycles and on days 9 to 12 for cycles 1 and 2 only plus bortezomib (1.3 mg/m2 IV on days 1, 4, 8, and 11) repeated every 3 weeks for 4 cycles as induction therapy prior to autologous stem-cell transplantation has been evaluated in newly diagnosed multiple myeloma patients in randomized, phase 3 studies.[49477] [49745]
40 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 plus bortezomib (1.3 mg/m2 IV on days 1, 4, 8, and 11) and thalidomide (100 mg orally daily for the first 14 days during cycle 1 only, and then 200 mg orally daily thereafter). Regimen is known as the VTD regimen. Repeated every 21 days for 3 cycles prior to a double (tandem) autologous stem-cell transplant (ASCT). This regimen was studied in a multicenter, randomized, phase 3 study. Patients randomized to induction therapy with VTD also received two 35-day consolidation cycles with VTD (bortezomib 1.3 mg/m2 on days 1, 8, 15, and 22 plus thalidomide 100 mg orally daily and dexamethasone 40 mg orally on days 1, 2, 8, 9, 15, 16, 22, and 23) following the second transplantation. Patients also received maintenance therapy with dexamethasone 40 mg orally on days 1 to 4 every 28 days until relapse or disease progression.[49746] Additionally in a randomized, phase 3 study, dexamethasone 40 mg orally daily on days 1 to 4 and 9 to 12 plus bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11) and thalidomide (200 mg orally daily after dose escalation as follows in the first cycle: thalidomide 50 mg/day on days 1 to 14 and 100 mg/day on days 15 to 28) repeated every 4 weeks for 6 cycles prior to an ASCT was studied. In this study, patients who received up to 3 years of maintenance therapy (starting 3 months after ASCT) with bortezomib (1.3 mg/m2 IV on days 1, 4, 8, and 11 repeated every 3 months) plus thalidomide (100 mg/day) had significantly improved 2-year progression-free survival compared with thalidomide or interferon alfa-2b maintenance therapy.[49747]
40 mg orally daily on days 1 to 4, days 9 to 12, and days 17 to 20 or dexamethasone 40 mg orally daily on days 1 to 4 on all cycles and days 9 to 12 and days 17 to 20 of cycles 1 and 2 only, plus doxorubicin 9 mg/m2 IV daily and vincristine 0.4 mg IV daily on days 1 to 4 (VAD regimen) has been studied. Doxorubicin and vincristine were administered as a continuous IV infusion over 24 hours/day [49477] or as a daily IV infusion. Cycles were repeated every 4 weeks for 3 to 4 cycles as induction therapy prior to autologous stem-cell transplantation.[49477] [49478]
20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 repeated every 21 days on cycles 1 to 8 and then 20 mg orally on days 1, 2, 8, and 9 starting on cycle 9 in combination with pomalidomide (4 mg orally daily on days 1 to 14) and bortezomib was evaluated in a randomized, phase 3 trial (n = 559; the OPTIMISMM trial). Bortezomib was administered as follows: 1.3 mg/m2 IV or subcutaneously on days 1, 4, 8, and 11 on cycles 1 to 8 then 1.3 mg/m2 IV or subcutaneously on days 1 and 8 starting on cycle 9. Treatment cycles were repeated every 21 days until disease progression.[64412]
10 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 repeated every 21 days on cycles 1 to 8 and then 10 mg orally on days 1, 2, 8, and 9 starting on cycle 9 in combination with pomalidomide (4 mg orally daily on days 1 to 14) and bortezomib. Bortezomib was administered as follows: 1.3 mg/m2 IV or subcutaneously on days 1, 4, 8, and 11 on cycles 1 to 8 then 1.3 mg/m2 IV or subcutaneously on days 1 and 8 starting on cycle 9. Treatment cycles were repeated every 21 days until disease progression.[64412]
40 mg orally or IV on days 1, 2, 8, 9, 15, 16, 22, and 23 in induction cycles 1 and 2; 40 mg orally or IV on days 1 and 2 and 20 mg PO or IV on days 8, 9, 15, and 16 in induction cycles 3 and 4; and 20 mg orally or IV on days 1, 2, 8, 9, 15, and 16 for 2 consolidation cycles in combination with daratumumab, bortezomib, and thalidomide was evaluated in a multicenter, randomized, phase 3 trial (n = 1,085; the CASSIOPEIA trial).[65868] In this trial, dexamethasone was administered for up to four 28-day induction therapy cycles and two 28-day consolidation therapy cycles with daratumumab (16 mg/kg IV weekly in induction cycles 1 and 2 then 16 mg/kg IV every 2 weeks in induction cycles 3 and 4 and for both consolidation cycles; bortezomib 1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11 in each induction and consolidation cycle; and thalidomide 100 mg orally daily. Consolidation therapy was begun after hematopoietic reconstitution but not earlier than 30 days after transplant.[64528]
40 mg IV or orally on days 1, 8, 15, and 22 repeated every 28 days until disease progression. Give in combination with isatuximab 10 mg/kg (actual body weight) IV on days 1, 8, 15, and 22 on cycle 1 and isatuximab 10 mg/kg (actual body weight) IV on days 1 and 15 starting on cycle 2 and pomalidomide 4 mg orally daily on days 1 to 21. The scheduled dexamethasone dose should be given prior to isatuximab and pomalidomide on days these drugs are given together.[65868] [65066] At a median follow-up time of 11.6 months, the median progression-free survival time (evaluated by an independent response committee) was significantly improved in patients with relapsed or refractory multiple myeloma who received isatuximab, pomalidomide, and low-dose dexamethasone compared with pomalidomide and low-dose dexamethasone alone (11.5 months vs. 6.5 months; hazard ratio (HR) = 0.596; 95% CI, 0.44 to 0.81; p = 0.001) in a multinational, randomized, phase 3 trial (the ICARIA-MM trial; n = 307). Patients (median age, 67 years) in this study had received a median of 3 prior therapies including lenalidomide and a proteasome inhibitor; 56% of patients had previously received an autologous stem-cell transplantation.[65070] At a second interim analysis (median follow-up, 35.3 months), the median overall survival time was 24.6 months in patients who received isatuximab, pomalidomide, and dexamethasone compared with 17.7 months in patients who received pomalidomide and dexamethasone (HR = 0.76; 95% CI, 0.57 to 1.01). Subsequent therapy was given at disease progression in 60% and 72% of patients in the isatuximab-containing and control arms, respectively. Of patients who received subsequent therapy, fewer patients received daratumumab in the isatuximab-containing arm (24% vs. 58%).[67409]
20 mg IV or orally on days 1, 8, 15, and 22 repeated every 28 days until disease progression. Give in combination with isatuximab 10 mg/kg (actual body weight) IV on days 1, 8, 15, and 22 on cycle 1 and isatuximab 10 mg/kg (actual body weight) IV on days 1 and 15 starting on cycle 2 and pomalidomide 4 mg orally daily on days 1 to 21. The scheduled dexamethasone dose should be given prior to isatuximab and pomalidomide on days these drugs are given together.[65868] [65066] At a median follow-up time of 11.6 months, the median progression-free survival was significantly improved in patients with relapsed or refractory multiple myeloma who received isatuximab, pomalidomide, and low-dose dexamethasone compared with pomalidomide and low-dose dexamethasone alone (11.5 months vs. 6.5 months; hazard ratio = 0.596; 95% CI, 0.44 to 0.81; p = 0.001) in a multinational, randomized, phase 3 trial (the ICARIA-MM trial; n = 307). Patients (median age, 67 years) in this study had received a median of 3 prior therapies including lenalidomide and a proteasome inhibitor; 56% of patients had previously received an autologous stem-cell transplantation.[65070]
20 mg PO/IV on days 1, 2, 8, 9, 15, and 16 and 40 mg PO/IV on day 22 repeated every 28 days in combination with IV carfilzomib (20 mg/m2 and 56 mg/m2 twice weekly regimen) and IV daratumumab until disease progression or unacceptable toxicity. Alternatively, dexamethasone may be given as follows: 20 mg PO/IV on days 1, 2, 8, 9, 15, 16, 22, and 23 in cycles 1 and 2; 20 mg PO/IV on days 1, 2, 15, and 16 and 40 mg PO/IV on days 8 and 22 in cycles 3, 4, 5, and 6; and 20 mg PO/IV on days 1 and 2 and 40 mg PO/IV on days 8, 15, and 22 in cycles 7 and beyond in combination with IV carfilzomib (20 mg/m2 and 70 mg/m2 once weekly regimen) and IV daratumumab until disease progression or unacceptable toxicity. Treatment cycles are repeated every 28 days. Give dexamethasone 30 minutes to 4 hours prior to the carfilzomib dose and 1 to 3 hours prior to daratumumab.[65868] [51306] At a median follow-up time of about 17 months, the median progression-free survival was significantly improved in patients with relapsed or refractory multiple myeloma who received carfilzomib 20 mg/m2 and 56 mg/m2 twice weekly regimen, daratumumab, and dexamethasone compared with carfilzomib and dexamethasone alone (median time not reached vs. 15.8 months; hazard ratio = 0.63; 95% CI, 0.46 to 0.85; p = 0.0027) in a multicenter, randomized (2:1), open-label, phase 3 trial (n = 466; the CANDOR trial).[65854] At a median follow-up time of 16.6 months (range, 0.5 to 27.4 months), the overall response rate was 84% (complete response rate, 33%) in patients with relapsed or refractory multiple myeloma who received carfilzomib 20 mg/m2 and 70 mg/m2 once weekly regimen, daratuzumab, and dexamethasone in a multicenter, multi-arm, phase 1b trial (n = 85; EQUULEUS trial).[65855]
20 mg PO/IV on days 1 and 2 of cycle 1 only and then 20 mg PO/IV weekly in combination with IV carfilzomib and IV daratumumab until disease progression or unacceptable toxicity. Treatment cycles are repeated every 28 days. Give dexamethasone 30 minutes to 4 hours prior to the carfilzomib dose and 1 to 3 hours prior to daratumumab.[65868] [51306] At a median follow-up time of about 17 months, the median progression-free survival was significantly improved in patients with relapsed or refractory multiple myeloma who received carfilzomib 20 mg/m2 and 56 mg/m2 twice weekly regimen, daratumumab, and dexamethasone compared with carfilzomib and dexamethasone alone (median time not reached vs. 15.8 months; hazard ratio = 0.63; 95% CI, 0.46 to 0.85; p = 0.0027) in a multicenter, randomized (2:1), open-label, phase 3 trial (n = 466; the CANDOR trial).[65854] At a median follow-up time of 16.6 months (range, 0.5 to 27.4 months), the overall response rate was 84% (complete response rate, 33%) in patients with relapsed or refractory multiple myeloma who received carfilzomib 20 mg/m2 and 70 mg/m2 once weekly regimen, daratuzumab, and dexamethasone in a multicenter, multi-arm, phase 1b trial (n = 85; EQUULEUS trial).[65855]
20 mg on days 1, 2, 8, 9, 15, and 16 repeated every 21 days on cycles 1, 2, 3, and 4 followed by high-dose chemotherapy and an autologous stem-cell transplant and then 2 additional cycles of dexamethasone 20 mg on days 1, 2, 8, 9, 15, and 16 repeated every 21 days (cycles 5 and 6) plus lenalidomide 25 mg orally daily on days 1 to 14 and bortezomib 1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11 repeated every 21 days for 6 cycles (VRd regimen) with daratumumab was evaluated in a randomized, phase 2 trial (the GRIFFIN trial; n = 207). Daratumumab treatment consisted of 16 mg/kg IV on days 1, 8, and 15 repeated every 21 days on cycles 1, 2, 3, and 4 and 16 mg/kg IV day 1 repeated every 21 days on cycles 5 and 6. Maintenance therapy was given for up to 2 years and consisted of daratumumab 16 mg/kg IV on day 1 repeated every 4 or 8 weeks and lenalidomide 10 mg orally daily on days 1 to 21 (increased to 15 mg after 3 cycles if tolerated) repeated every 28 days.[66069]
20 mg PO on days 1 and 2 in combination with selinexor 100 mg orally on day 1 once weekly and bortezomib 1.3 mg/m2 subcutaneously on day 1 once weekly for 4 weeks followed by 1 week off; repeat cycles until disease progression.[64399] Treatment with a once-weekly regimen of selinexor plus bortezomib, and dexamethasone (SVd regimen) led to a significantly improved median progression-free survival time compared with bortezomib and dexamethasone (13.93 months vs. 9.46 months; hazard ratio (HR) = 0.7; 95% CI, 0.53 to 0.93) in a randomized, phase 3 trial (n = 402; the Boston trial). At a median follow-up of 17.3 months, the median overall survival (OS) time was not significantly improved in the SVd arm (HR = 0.84; 95% CI, 0.57 to 1.23); however, OS data was not mature at the time of this analysis. Patients (median age, 67 years) in this trial had received a median of 2 prior regimens (range, 1 to 2 regimens) and approximately 70% of patients had received prior bortezomib therapy; 35% of patients had previously received a stem-cell transplant.[66186]
40 mg orally or IV on days 1, 8, 15, and 22 in combination with melphalan flufenamide 40 mg IV on day 1 repeated every 28 days until disease progression. Treatment with melphalan flufenamide plus dexamethasone resulted in an overall response rate of 23.7% in 97 patients with multiple myeloma who had received 4 or more previous lines of therapy and were refractory to at least 1 proteasome inhibitor, 1 immunomodulatory agent, and a CD38-directed monoclonal antibody in a nonrandomized phase 2 trial (the HORIZON trial). No patient achieved a complete response. In this trial, the median duration of response was 4.2 months. Patients (median age, 65 years; range, 35 to 86 years) had received a median of 6 prior regimens (range, 4 to 12 regimens); 75% of patients had alkylator refractory disease and 70% of patients had previously received a stem-cell transplant.[66471]
20 mg orally or IV on days 1, 8, 15, and 22 in combination with melphalan flufenamide 40 mg IV on day 1 repeated every 28 days until disease progression. Treatment with melphalan flufenamide plus dexamethasone resulted in an overall response rate of 23.7% in 97 patients with multiple myeloma who had received 4 or more previous lines of therapy and were refractory to at least 1 proteasome inhibitor, 1 immunomodulatory agent, and a CD38-directed monoclonal antibody in a nonrandomized phase 2 trial (the HORIZON trial). No patient achieved a complete response. In this trial, the median duration of response was 4.2 months. Patients (median age, 65 years; range, 35 to 86 years) had received a median of 6 prior regimens (range, 4 to 12 regimens); 75% of patients had alkylator refractory disease and 70% of patients had previously received a stem-cell transplant.[66471]
20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 in combination with isatuximab (cycle 1: 10 mg/kg IV on days 1, 8, 15, and 22; cycle 2 and beyond: 10 mg/kg IV on days 1 and 15) and carfilzomib (cycle 1: 20 mg/m2 IV on days 1 and 2 and then 56 mg/m2 on days 8, 9, 15, and 16; cycle 2 and beyond: 56 mg/m2 IV on days 1, 2, 8, 9, 15, and 16). Repeat treatment cycles every 28 days until disease progression. Give IV dexamethasone prior to isatuximab and/or carfilzomib on days these agents are given on the same day and then give dexamethasone orally for other scheduled doses.[65066] [51306] At a median follow-up time of 44 months, the median progression-free survival time (evaluated by an independent review committee) was significantly improved in patients with relapsed or refractory multiple myeloma who received isatuximab, carfilzomib, and dexamethasone compared with carfilzomib and dexamethasone alone (35.7 months vs. 19.2 months; hazard ratio (HR) = 0.58; 95% CI, 0.42 to 0.79) in a prespecified interim analysis of a multinational, randomized, phase 3 trial (the IKEMA trial; n = 302). Median overall survival (OS) was not significantly improved in isatuximab-treated patients (HR = 0.78; 95% CI, 0.54 to 1.12); however, OS data were not mature at this analysis. Patients (median age, 65 years) in the isatuximab-treated arm had received a median of 2 (range, 1 to 4) prior therapies and 49.7% of patients were refractory to the last regimen.[69167]
40 mg orally on days 1, 8, 15, and 22 in combination with ixazomib 4 mg orally on days 1, 8, and 15 and lenalidomide 25 mg orally daily on days 1 through 21. Repeat treatment cycles every 28 days until disease progression.[60335] The median progression-free survival time was significantly improved with ixazomib plus lenalidomide and dexamethasone compared with placebo plus lenalidomide and dexamethasone (20.6 months vs. 14.7 months; hazard ratio (HR) = 0.74; 95% CI, 0.59 to 0.94; p = 0.01) in patients with relapsed and/or refractory multiple myeloma who had received 1 to 3 prior therapies in a multinational, randomized, double-blind, phase 3 trial (n = 722; TOURMALINE-MM1 trial). At a median follow-up time of 85 months, the median overall survival time was not significantly improved in the ixazomib-containing arm (53.6 months vs. 51.6 months; HR = 0.939; 95%CI, 0.784 to 1.125). In this trial, subsequent lines of therapy were given in 71.7% and 69.9% of patients who received ixazomib plus lenalidomide and dexamethasone (median, 2 subsequent therapies; range, 1 to 9) and lenalidomide and dexamethasone (median, 3 subsequent therapies; range, 1 to 12), respectively. The median age of patients in this study was 66 years (range, 30 to 91 years); prior therapy included a stem-cell transplant in 57% of patients, proteasome inhibitor therapy in 70% of patients, and immunomodulatory drug therapy in 55% of patients. Thromboprophylaxis was recommended for all patients.[60856] [66762]
40 mg orally once weekly (on days 1, 8, 15, and 22) repeated every 28 days until disease progression; give in combination with daratumumab/hyaluronidase (1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), every other week on weeks 9 to 24 (8 doses), and then every 4 weeks starting on week 25 until disease progression) and pomalidomide (4 mg PO daily on days 1 to 21 repeated every 28 days).[65366] At a median follow-up time of 16.9 months, the median progression-free survival time was significantly improved in patients with relapsed or refractory multiple myeloma who received daratumumab/hyaluronidase plus pomalidomide and dexamethasone compared with pomalidomide and dexamethasone alone (12.4 months vs. 6.9 months; hazard ratio (HR) = 0.63; 95% CI, 0.47 to 0.85) in a randomized, phase 3 (APOLLO) trial (n = 304). At a median follow-up of 39.6 months, the median overall survival time was not significantly improved in the daratumumab/hyaluronidase-containing arm (34.4 months vs. 23.7 months; HR = 0.82; 95% CI, 0.61 to 1.11). In this trial, eligible patients had received at least 1 previous line of therapy with both lenalidomide and a proteasome inhibitor, had a partial response or better to one or more previous lines of anti-myeloma therapy, and were refractory to lenalidomide if they had received only 1 previous line of treatment. Patients (median age, 67 years; range, 42 to 86 years) in the daratumumab/hyaluronidase arm had received a median of 2 (range, 1 to 5) prior therapies; 60% of patients had received a prior autologous stem-cell transplantation.[66809] [70310]
20 mg orally once weekly (on days 1, 8, 15, and 22) repeated every 28 days until disease progression; give in combination with daratumumab/hyaluronidase (1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), every other week on weeks 9 to 24 (8 doses), and then every 4 weeks starting on week 25 until disease progression) and pomalidomide (4 mg PO daily on days 1 to 21 repeated every 28 days).[65366] At a median follow-up time of 16.9 months, the median progression-free survival time was significantly improved in patients with relapsed or refractory multiple myeloma who received daratumumab/hyaluronidase plus pomalidomide and dexamethasone compared with pomalidomide and dexamethasone alone (12.4 months vs. 6.9 months; hazard ratio (HR) = 0.63; 95% CI, 0.47 to 0.85) in a randomized, phase 3 (APOLLO) trial (n = 304). At a median follow-up of 39.6 months, the median overall survival time was not significantly improved in the daratumumab/hyaluronidase-containing arm (34.4 months vs. 23.7 months; HR = 0.82; 95% CI, 0.61 to 1.11). In this trial, eligible patients had received at least 1 previous line of therapy with both lenalidomide and a proteasome inhibitor, had a partial response or better to one or more previous lines of antimyeloma therapy, and were refractory to lenalidomide if they had received only 1 previous line of treatment. Patients (median age, 67 years; range, 42 to 86 years) in the daratumumab/hyaluronidase arm had received a median of 2 (range, 1 to 5) prior therapies; 60% of patients had received a prior autologous stem-cell transplantation.[66809] [70310]
40 mg PO or IV per week in combination with carfilzomib and daratumumab/hyaluronidase; treatment cycles are repeated every 28 days until disease progression or unacceptable toxicity. Dexamethasone dosing day(s)/schedule differ with twice weekly or once weekly carfilzomib regimens. Give dexamethasone 30 minutes to 4 hours prior to the carfilzomib. Give the treatment dexamethasone dose as the premedication steroid when these drugs are scheduled on the same day as daratumumab; hyaluronidase. With carfilzomib 20 mg/m2 and 56 mg/m2 twice weekly regimen, give dexamethasone 20 mg PO/IV on days 1, 2, 8, 9, 15, 16, 22 and 23 on cycles 1 and 2 and then 20 mg PO/IV on days 1, 2, 8, 9, 15, and 16 and 40 mg PO/IV on day 22 on subsequent cycles. With carfilzomib 20 mg/m2 and 70 mg/m2 once weekly regimen, give dexamethasone 20 mg PO/IV on days 1, 2, 8, 9, 15, 16, 22, and 23 on cycles 1 and 2; 20 mg PO/IV on days 1, 2, 15, and 16 and 40 mg PO/IV on days 8 and 22 on cycles 3, 4, 5, and 6; and 20 mg PO/IV on days 1 and 2 and 40 mg PO/IV on days 8, 15, and 22 on cycles 7 and beyond. In patients with a BMI of less than 18.5 who received the carfilzomib once weekly regimen, give a reduced dexamethasone dosage of 20 mg PO/IV on days 1 and 2 of cycle 1 then 20 mg PO/IV weekly. Daratumumab; hyaluronidase is administered as follows: 1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), 1,800 mg daratumumab and 30,000 units hyaluronidase every 2 weeks on weeks 9 to 24 (8 doses), and then 1,800 mg daratumumab and 30,000 units hyaluronidase every 4 weeks starting on week 25 until disease progression. The overall response rate was 84.8% in 66 patients with relapsed or refractory multiple myeloma who received carfilzomib (20 mg/m2 and 70 mg/m2 once weekly regimen), daratumumab/hyaluronidase, and dexamethasone in a multicohort, phase 2 trial (the PLEIADES trial). The stringent complete response rate was 16.7% and the complete response rate was 21.2%. At a median follow-up time of 9.2 months, the median duration of response was not reached. Patients (median age, 61 years; range, 42 to 84 years) in this trial had received at least 1 previous therapy line that contained lenalidomide; 79% of patients had a prior stem-cell transplant.[51306] [65366]
20 mg PO/IV on days 1 and 2 of week 1 and then 20 mg PO/IV once weekly in combination with carfilzomib and daratumumab/hyaluronidase; treatment cycles are repeated every 28 days until disease progression or unacceptable toxicity. Give dexamethasone 30 minutes to 4 hours prior to the carfilzomib. Carfilzomib is administered as either a 20 mg/m2 and 56 mg/m2 twice weekly or a 20 mg/m2 and 70 mg/m2 once weekly regimen. Give the treatment dexamethasone dose as the premedication steroid when these scheduled on the same day as daratumumab; hyaluronidase. Daratumumab; hyaluronidase is administered as follows: 1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), 1,800 mg daratumumab and 30,000 units hyaluronidase every 2 weeks on weeks 9 to 24 (8 doses), and then 1,800 mg daratumumab and 30,000 units hyaluronidase every 4 weeks starting on week 25 until disease progression. The overall response rate was 84.8% in 66 patients with relapsed or refractory multiple myeloma who received carfilzomib (20 mg/m2 and 70 mg/m2 once weekly regimen), daratumumab/hyaluronidase, and dexamethasone in a multicohort, phase 2 trial (the PLEIADES trial). The stringent complete response rate was 16.7% and the complete response rate was 21.2%. At a median follow-up time of 9.2 months, the median duration of response was not reached. Patients (median age, 61 years; range, 42 to 84 years) in this trial had received at least 1 previous therapy line that contained lenalidomide; 79% of patients had a prior stem-cell transplant.[51306] [65366]
20 mg (IV on days of isatuximab infusions and PO on the other days) on days 1, 2, 4, 5, 8, 9, 11, 12, 15, 22, 23, 25, 26, 29, 30, 32, and 33 of cycles 1 to 4 (42-day cycles) and on days 1, 8, 15, and 22 starting on cycle 5 (28-day cycles). Give in combination with isatuximab 10 mg/kg (actual body weight) IV on days 1, 8, 15, 22, and 29 of cycle 1 (42-day cycle), on days 1, 15, and 29 of cycles 2, 3, and 4 (42-day cycles), on days 1 and 15 of cycles 5 to 17 (28-day cycles), and on day 1 of cycles 18 and beyond (28-day cycles); bortezomib 1.3 mg/m2 subcutaneously on days 1, 4, 8, 11, 22, 25, 29, and 32 of cycles 1 to 4 (42-day cycles); lenalidomide 25 mg PO daily on days 1 to 14 and on days 22 to 35 of cycles 1 to 4 (42-day cycles) and on days 1 to 21 starting on cycle 5 (28-day cycles). Administer dexamethasone (as part of isatuximab premedication and as part of the scheduled regimen dose) before isatuximab, bortezomib, and lenalidomide.[65066] [65868] At a median follow-up of 60 months, the median progression-free survival (PFS) time was significantly improved in patients with newly diagnosed multiple myeloma not eligible for autologous stem cell transplant who received isatuximab, bortezomib, lenalidomide, and dexamethasone compared with bortezomib, lenalidomide, and dexamethasone alone (not reached vs. 54.3 months) in a randomized, phase 3 study (IMROZ); the improvement in PFS represented a 40% reduction in the risk of disease progression or death. Crossover from the control arm to the experimental arm was allowed after cycle 4 at the investigator's discretion.[65066] [71276]
30 mg/day PO for 7 days, followed by doses of 4 to 12 mg PO every other day for 1 month have been shown to be effective. Controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations, they do not show that they affect the ultimate outcome or natural history of the disease.[30011]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response.
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response.
Initially, 0.75 to 9 mg/day PO, given in 2 to 4 divided doses. Adjust according to patient response.[30011] Because of the potential complications of steroid use, steroids should be used selectively and in the lowest dose possible for the shortest duration as possible.[64393] [64397]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO given in 3 to 4 divided doses is the FDA-approved general dosage range.[54286] Adjust according to patient response. Because of the potential complications of steroid use, steroids should be used selectively and in the lowest dose possible for the shortest duration as possible.[64393] [64397]
Initially, 0.5 to 9 mg/day IV or IM, in 2 to 4 divided doses. Adjust according to patient response.[60760] Because of the potential complications of steroid use, steroids should be used selectively and in the lowest dose possible for the shortest duration as possible.[64393] [64397]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM given in 3 to 4 divided doses is the FDA-approved general dosage range.[54285] [54286] Adjust according to patient response. Because of the potential complications of steroid use, steroids should be used selectively and in the lowest dose possible for the shortest duration as possible.[64393] [64397]
1 to 2 drops into affected eye(s) every hour during the day and every 2 hours during the night, initially. Reduce dose to 1 drop into affected eye(s) every 4 hours when a favorable response occurs, and then to 1 drop into affected eye(s) 3 to 4 times daily.[54348]
1 to 2 drops into affected eye(s) every hour for severe disease and every 4 to 6 hours for mild disease. Taper dose to discontinuation as inflammation subsides.[61633]
1 to 2 drops into affected eye(s) every hour for severe disease and every 4 to 6 hours for mild disease. Taper dose to discontinuation as inflammation subsides.[61633]
0.75 to 9 mg/day PO in 2 to 4 divided doses, initially. Adjust according to individual response.[54286]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day PO in 3 to 4 divided doses, initially. Adjust according to individual response.[54286]
0.5 to 9 mg/day IV or IM in 2 to 4 divided doses, initially. Adjust according to individual response.[54285] [54286]
0.02 to 0.3 mg/kg/day or 0.6 to 9 mg/m2/day IV or IM in 3 to 4 divided doses. Adjust according to individual response.[54285] [54286]
0.4 mg in the lower lacrimal punctum into the canaliculus as a single dose. A single insert releases a 0.4 mg dose od dexamethasone for up to 30 days after insertion.[63796]
0.4 mg in the lower lacrimal punctum into the canaliculus as a single dose. A single insert releases a 0.4 mg dose od dexamethasone for up to 30 days after insertion.[63796]
0.7 mg implant by intravitreal injection into affected eye(s).[41921] [71021]
0.7 mg implant by intravitreal injection into affected eye(s).[71021] [71024]
1 to 2 drops into both eyes 4 times daily, initially. Reduce dose to 1 to 2 drops into both eyes twice daily after 1 to 2 weeks if positive response in signs and/or symptoms and start cyclosporine, then taper or discontinue steroid therapy after 2 to 4 weeks. Consider extending duration to 4 weeks if no response at 2 weeks, especially in individuals with moderate to severe disease.[68206] [71210]
0.7 mg implant by intravitreal injection in the affected eye(s).[41921] Guidelines recommend intravitreous steroids as a second-line alternative treatment for central-involved diabetic macular edema (CIDME). Steroid therapies are associated with inferior visual acuity outcomes and increased rate of cataracts and glaucoma when compared against intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents.[61821] [64926]
0.7 mg implant by intravitreal injection in the affected eye(s).[41921] Retreatment will generally be performed after 3 to 4 months with a mean of approximately 2 to 3 injections/year. Guidelines suggest switching to a steroid in nonresponders who have already been treated with anti-vascular endothelial growth factor (VEGF) (after 3 to 6 injections, depending on the specific response of each patient) is reasonable. Steroids may be considered as a first-line therapy for patients who have a recent history of a major cardiovascular event or those who are unwilling to come for monthly injections (and/or monitoring) in the first 6 months of therapy; however, intraocular pressure still needs to be monitored every 2 to 8 weeks after dexamethasone implant injection.[67516]
0.005 mL of dexamethasone 9% (equivalent to 517 mcg) as a single dose as directed, intraocularly in the posterior chamber at the end of surgery.[48640]
Place the insert containing 0.4 mg of dexamethasone into the lower lacrimal canaliculus, just below the punctal opening. A single insert releases a 0.4 mg dose for up to 30 days following insertion.[63796]
Place the insert containing 0.4 mg of dexamethasone into the lower lacrimal canaliculus, just below the punctal opening. A single insert releases a 0.4 mg dose for up to 30 days following insertion.[63796]
0.5 mg PO once daily at bedtime, administered on cycle days 3 to 12, days 5 to 9, or starting on day 5 and continuing through conception, in combination with clomiphene (doses ranging from 50 to 200 mg/day) has been studied.[32778] [32779] [32781] Alternatively, dexamethasone 2 mg PO once daily on cycle days 5 to 14 in combination with clomiphene 200 mg/day or dexamethasone 1 mg PO twice daily on cycle days 3 to 12 in combination with clomiphene 100 mg/day PO has also been studied; HCG was administered to augment ovulation.[32780] [32782] Optimal timing and dose of dexamethasone is not clear and has varied from study to study. Combination therapy has been shown to increase ovulation rates (range, 75% to 100%) and pregnancy rates (range, 38% to 74%) in women with both normal and elevated DHEA-S concentrations and in those women with or without polycystic ovary syndrome (PCOS). A Cochrane's review indicates that dexamethasone-clomiphene combination is one of the few adjunctive therapies for infertility that has been shown to improve pregnancy rates (fixed OR 11.3, 95% CI 5.3 to 24; NNT 2.7, 95% CI 2.1 to 3.6) [32783]; the 2 studies in this review used differing doses of 0.5 mg PO at bedtime on days 5 to 9 or 2 mg PO/day on days 5 to 14.[32778] [32782] Several theories on the mechanism of dexamethasone in infertility exist. One theory is that dexamethasone enhances folliculogenesis by suppressing adrenal androgen hypersecretion, which should augment the actions of clomiphene. Dexamethasone may increase FSH concentrations thereby facilitating folliculogenesis. Finally, dexamethasone may decrease the elevated LH concentrations in patients with PCOS.
4 mg PO every 6 hours until symptoms resolve.[72681] [72682]
0.15 mg/kg/dose (Max: 4 mg/dose) PO every 6 hours until symptoms resolve.[56398] [72681] [72682]
4 mg IV or IM every 6 hours until symptoms resolve.[72681] [72682]
0.15 mg/kg/dose (Max: 4 mg/dose) IV or IM every 6 hours until symptoms resolve.[56398] [72681] [72682]
8 mg PO once, then 4 mg PO every 6 hours until symptoms resolve. May add acetazolamide.[72681] [72682]
0.15 mg/kg/dose (Max: 4 mg/dose) PO every 6 hours until symptoms resolve. May add acetazolamide.[56398] [72681] [72682]
8 mg IV or IM once, then 4 mg IV or IM every 6 hours until symptoms resolve. May add acetazolamide.[72681] [72682]
0.15 mg/kg/dose (Max: 4 mg/dose) IV or IM every 6 hours until symptoms resolve. May add acetazolamide.[56398] [72681] [72682]
2 to 4 mg IV once for established post-operative nausea/vomiting (PONV), per treatment guidelines; readministration of longer-acting drugs, such as dexamethasone, is not recommended.[57398] If PONV prophylaxis was either inadequate or not initially given, dexamethasone is an appropriate rescue treatment option if not initially used for PONV prophylaxis. Of note, the 5-HT3 antagonists are the only class of drugs that have been adequately studied for the treatment of established PONV.[57398]
4 to 5 mg IV at anesthesia induction is recommended by treatment guidelines for patients at an increased risk for post-operative nausea and vomiting (PONV); administration at induction rather than at the end of surgery is preferred. Some studies suggest that 8 mg IV is associated with a dose-dependent increase in quality of recovery, including reduced fatigue, postoperative pain, and need for opioid analgesia; however, further confirmation is necessary before larger doses are universally recommend. Safety data regarding the perioperative use of dexamethasone point to a possible increased risk of wound infection and/or increased blood glucose in some patients. A single dexamethasone dose (4 to 8 mg IV) is, however, considered safe for PONV prophylaxis. For patients with labile glucose control, dexamethasone use is relatively contraindicated.[57398]
0.15 to 1 mg/kg/dose IV (Max: 8 to 25 mg/dose IV) given as a single intraoperative dose reduces the incidence of postoperative nausea/vomiting in the first 24 hours, improves postoperative pain control, and decreases the time to resumption of soft/solid diet without adverse effects and is recommended in patients undergoing tonsillectomy.[54553] [54554] A lower dose of 0.015 mg/kg/dose (Max: 5 mg/dose) in combination with ondansetron 0.1 mg/kg/dose (Max: 4 mg) is recommended first-line for postoperative vomiting prophylaxis in children by the Society for Ambulatory Anesthesiology.[57398]
2 mg PO every 6 hours or 4 mg PO every 12 hours starting the day of ascent and continuing for 2 to 4 days after reaching the target altitude or until descent is initiated. Tapering is not required if use is limited to 7 days or less but may be necessary with longer duration. May consider 4 mg PO every 6 hours for very high-risk situations (e.g., military or search and rescue personnel being airlifted to altitudes higher than 3,500 meters with immediate performance of physical activity).[72681] [72682]
8 mg PO every 12 hours starting the day before ascent and continuing for 4 to 7 days after reaching the target altitude or until descent is initiated. Tapering is not required if use is limited to 7 days or less but may be necessary with longer duration.[72681] [72682]
Due to the lack of consistent efficacy data and the high risk of adverse effects, the American Academy of Pediatrics does not recommend systemic corticosteroids for the management of bronchiolitis in any setting.[58442] However, other authors state corticosteroids may be beneficial in severely ill or mechanically ventilated patients.[54551] One randomized trial of 800 infants seen in the emergency department used 1 mg/kg PO once (Max: 10 mg/dose) followed by 0.6 mg/kg/dose PO once daily (Max: 10 mg/dose) for 5 days. Dexamethasone in combination with nebulized epinephrine was effective in reducing hospital admissions by day 7 of illness compared to treatment with dexamethasone alone, epinephrine alone, or placebo.[39327] In a study of 200 infants (median age 3.5 months) with an asthma risk, as determined by eczema or a family history of asthma in a first-degree relative, dexamethasone 1 mg/kg (single dose) PO then 0.6 mg/kg/dose PO once daily for 4 more days was administered with salbutamol. In infants receiving dexamethasone with salbutamol, the time to readiness for discharge was 18.6 hours vs. 27.1 hours in patients not receiving dexamethasone (p = 0.015).[56911] In contrast, 1 mg/kg/dose PO (Max: 12 mg/dose) given as a single dose did not reduce hospitalization rates, Respiratory Assessment Change Scores (RACS), length of hospitalization for those patients who required admission, or subsequent hospitalizations within 7 days compared to placebo in another large, randomized trial (n = 600).[33394]
Due to the lack of consistent efficacy data and the high risk of adverse effects, the American Academy of Pediatrics does not recommend systemic corticosteroids for the management of bronchiolitis in any setting.[58442] However, other authors state corticosteroids may be beneficial in severely ill or mechanically ventilated patients.[54551] 0.15 mg/kg/dose IV every 6 hours for 48 hours with the first dose administered within 24 hours of mechanical ventilation was used in patients with respiratory syncytial virus. In a post hoc analysis of patients with bronchiolitis (n = 39), the mean duration of mechanical ventilation and of supplemental oxygen were significantly shorter in patients receiving dexamethasone compared to those receiving placebo (4.9 and 7.7 days vs. 9.2 and 11.3 days, respectively); no differences were seen in the length of intensive care unit or hospital stay.[54547]
20 mg IV on day 1 in combination with rituximab 375 mg/m2 IV on day 1 and cyclophosphamide 100 mg/m2 orally twice daily on days 1 to 5 (total dose of 1,000 mg/m2/cycle) repeated every 21 days for 6 cycles was evaluated in a single-arm, phase II trial.[61073]
40 mg IV on days 1, 8, 15, and 22 in cycles 2 and 5 in combination with bortezomib and rituximab was evaluated in a nonrandomized phase II trial. Bortezomib was given as follows: 1.3 mg/m2 IV on days 1, 4, 8, and 11 for the first 21-day cycle (cycle 1) then 1.6 mg/m2 IV on days 1, 8, 15, and 22 repeated every 35 days for 4 additional cycles (cycles 2, 3, 4, and 5). Rituximab was given as 375 mg/m2 IV on days 1, 8, 15, and 22 in cycles 2 and 5 (for 8 total doses). All patients received premedication with acetaminophen 1,000 mg PO and diphenhydramine 50 mg IV prior to rituximab and herpes zoster prophylaxis with valacyclovir or acyclovir.[61131]
Dexamethasone in combination with lenalidomide (15 mg PO daily on days 1 to 21) and cyclophosphamide repeated every 28 days has been evaluated in nonrandomized, phase II studies. Treatment duration, drug dosages of cyclophosphamide and dexamethasone, and thromboprophylaxis agents/recommendations varied in these studies.[61321] [61322] [61323] In one study, 12 cycles of dexamethasone (20 mg PO on days 1, 2, 3, 4, 9, 10, 11, and 12 for 6 cycles; then 20 mg PO on days 1, 2, 3, and 4 for an additional 6 cycles), lenalidomide, and cyclophosphamide (300 mg/m2 IV on days 1 and 8 for 6 cycles; then 300 mg/m2 IV on day 1 for an additional 6 cycles) were given and then maintenance therapy with lenalidomide and dexamethasone was administered for 3 additional years or until disease progression. Patients with cardiac stage III had an upfront dose modification of dexamethasone.[61321] In another study, dexamethasone (40 mg PO on days 1, 8, 15, and 22), lenalidomide, and cyclophosphamide (500 mg PO on days 1, 8, and 15) therapy was given for a maximum of 9 cycles; treatment was discontinued after cycle 6 if a complete response or partial response/very good partial response plus organ response was obtained. In this study, patients with fluid retention over 3% of body weight despite optimal diuretic use received a lower dose of dexamethasone (20 mg once weekly).[61322] In a third study, cycles of dexamethasone (40 mg PO on days 1, 8, 15, and 22), lenalidomide, and cyclophosphamide (300 mg/m2 PO on days 1, 8, and 15) were continued until disease progression, unacceptable toxicity, or up to 2 years; however, cyclophosphamide was given for up to a maximum of 12 cycles only.[61323]
40 mg orally on days 1, 8, 15, and 22 in combination with lenalidomide (10 mg PO daily on days 1 to 21) and melphalan repeated every 28 days has been evaluated in nonrandomized studies. Treatment duration, the melphalan dosage, and thromboprophylaxis agents/recommendations varied in these studies.[61331] [61332] In one study, melphalan (0.18 mg/kg PO daily on days 1, 2, 3, and 4), lenalidomide, and dexamethasone therapy was given for a maximum of 9 cycles; single-agent lenalidomide was continued in responding patients.[61331] In another study, lenalidomide, melphalan (5 mg/m2 PO daily on days 1, 2, 3, and 4), and dexamethasone were continued until disease progression, unacceptable toxicity, or up to 12 cycles.[61332]
40 mg orally daily on days 1, 2, 3, and 4 repeated every 28 days on cycles 1 and 2 and then 40 mg orally daily on days 1, 2, 3, and 4 repeated every 35 days up to a maximum of 8 cycles in combination with bortezomib and melphalan (BMdex regimen) was evaluated in a multicenter, randomized, open-label, phase 3 trial (n = 109). Patients were evaluated for response after 3 and 6 cycles of therapy; patients with a partial response (PR) or better after cycle 3 received an additional 3 cycles of therapy. Patients with a complete response (CR) or a PR and organ response stopped treatment after cycle 6.[65948]
40 mg IV or PO in combination with bortezomib 1.3 mg/m2 subcutaneously and cyclophosphamide 300 mg/m2 (Max dose of 500 mg) IV or PO each given weekly on days 1, 8, 15, and 22 repeated every 28 days for a maximum of 6 cycles (VCd) plus up to 2 years of subcutaneous daratumumab; hyaluronidase (D-VCd) was evaluated in transplant eligible, newly diagnosed light-chain amyloidosis patients in a randomized, phase 3 trial (n = 388; the ANDROMEDA trial). The dose of dexamethasone was reduced to 20 mg in patients older than 70 years or who had a body mass index less than 18.5, hypervolemia, poorly controlled diabetes mellitus, or prior intolerance to steroid therapy. Daratumumab; hyaluronidase was administered as follows: 1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 8 (8 doses), 1,800 mg daratumumab and 30,000 units hyaluronidase every 2 weeks on weeks 9 to 24 (8 doses), and then 1,800 mg daratumumab and 30,000 units hyaluronidase every 4 weeks starting on week 25 until disease progression or for a maximum of 2 years.[65366] At a median follow-up time of 11.4 (range, 0.03 to 21.3) months, the hematologic complete response rate was significantly improved (53.3% vs. 18.1%; relative risk ratio = 2.9; 95% CI, 2.1 to 4.1; p less than 0.001) in patients who received D-VCd compared with VCd in the ANDROMEDA trial. The median time to hemCR was 60 and 85 days in the D-VCd and VCd arms, respectively.[66968]
10 mg PO once daily for 1 to 2 days.[67513]
0.6 mg/kg/dose (Max: 10 mg/dose) PO once daily for 1 to 2 days.[67513]
10 mg IM once daily for 1 to 2 days.[67513]
0.6 mg/kg/dose (Max: 10 mg/dose) IM once daily for 1 to 2 days.[67513]
2 mg PO every 6 hours. Taper dose based on clinical response and the duration of steroid therapy.[61515] [68189]
2 mg IV every 6 hours. Taper dose based on clinical response and the duration of steroid therapy.[61515] [68189]
6 to 8 mg PO divided into 3 daily doses starting 3 days before antiparasitics and continuing for the duration of therapy. Titrate based on clinical response. Taper over 6 to 8 weeks to avoid rebound symptoms.[63735] [69053] [69054] [69056] [69057]
0.1 to 0.2 mg/kg/day PO starting 3 days before antiparasitics and continuing for the duration of therapy. Titrate based on clinical response. Taper over 6 to 8 weeks after antiparasitic therapy is complete to avoid rebound symptoms.[63735] [69053] [69056] [69057]
4 mg PO every 6 hours for 24 to 48 hours. Glucocorticoids may stabilize and prevent osmotic disruption of the blood-brain barrier.[62924]
4 mg IV every 6 hours for 24 to 48 hours. Glucocorticoids may stabilize and prevent osmotic disruption of the blood-brain barrier.[62924]
10 mg IV every 12 hours for up to 48 hours antepartum, then 5 to 10 mg IV every 12 hours for 24 to 48 hours postpartum. Duration may be shortened or extended based on clinical response.[38667] [70966] [70967] [70968]
1 mg PO 4 times daily as a oral rinse.[70996] [70997] [70998]
2.5 to 10 mg PO once daily for 2 consecutive days every week until arrest of disease activity. May increase the dose by 2.5 mg/dose based on clinical response. Max: 10 mg/dose.[71057] [71058] [71069] [71070] [71074]
2.5 to 5 mg PO once daily for 2 consecutive days every week until arrest of disease activity. May increase the dose by 2.5 mg/dose based on clinical response. Max: 7.5 mg/dose.[71057] [71058] [71070] [71074]
1.6 to 19.2 mg by intratympanic injection as a single dose or every 3 to 7 days for up to 4 doses based on clinical response.[71188]
20 to 30 mg/day IV in 1 to 4 divided doses, starting after IV methylprednisolone, until the target partial oxygen saturation is achieved or for up to 14 days.[71529] [71530] [71532] [71533]
8 mg PO 3 times daily for 14 days, starting after IV methylprednisolone.[71529]
0.5 mg PO 3 times daily as an oral rinse.[71608] [71681]
Specific guidelines for systemic dosage adjustments in hepatic impairment are not available; it appears that no dosage adjustments are needed.
Specific guidelines for dosage adjustments in renal impairment are not available; it appears that no dosage adjustments are needed.
† Off-label indication







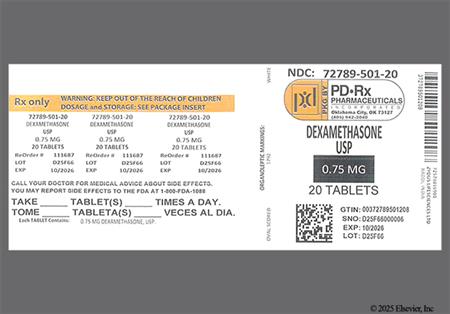


































Dexamethasone and its derivatives, dexamethasone sodium phosphate and dexamethasone acetate, are synthetic glucocorticoids used as anti-inflammatory or immunosuppressive agents. Dexamethasone is available as oral, parenteral, as well as topical ophthalmic and intraocular dosage forms. Dexamethasone is used for many conditions in adult and pediatric patients, including cerebral edema, prevention of transplant rejection, and many allergic, dermatologic, ophthalmic, and systemic inflammatory conditions. Systemic dexamethasone is usually selected for the management of cerebral edema because of its superior ability to penetrate the CNS. Dexamethasone is also commonly used in antiemetic regimens for chemotherapy patients and is included in the American Society of Clinical Oncology (ASCO) guideline-based dosage regimens.[63197] In general, prednisone is more commonly prescribed as an oral corticosteroid when systemic treatment is needed for most conditions. Dexamethasone has little to no mineralocorticoid activity and is therefore not used by itself in the management of adrenal insufficiency. Systemic corticosteroids may be added to other long-term maintenance medications in the management of uncontrolled severe persistent asthma. Once stabilization of asthma is achieved, regular attempts should be made to reduce or eliminate the use of systemic corticosteroids due to the side effects associated with chronic administration. Short courses of treatment may be used in the management of asthma exacerbations.[66299][69016]
Updates for coronavirus disease 2019 (COVID-19):
The National Institutes of Health (NIH) COVID-19 treatment guidelines have released recommendations for the use of corticosteroids that are based on disease severity.
Adult patients
Pediatric patients
The World Health Organization strongly recommends the use of systemic corticosteroids, including dexamethasone, in patients with severe or critical COVID-19; but suggests against use in patients with non-severe COVID-19.[65876]
For storage information, see the specific product information within the How Supplied section.
Dexamethasone Intensol (Oral Solution Concentrate)
Visually inspect parenteral products for particulate matter and discoloration prior to administration whenever solution and container permit.
The safety and effectiveness of epidural administration of corticosteroids have not been established, and corticosteroids are not approved for this use. Reports of serious medical and neurologic events, including death, have been associated with epidural routes of corticosteroid administration.[60760]
Direct IV injection:
Intermittent or continuous IV infusion:
Intra-articular, Soft tissue, or Intralesional injection
Ophthalmic solution or suspension:
Intraocular Administration
Dexycu Intraocular Suspension
Preparation of intraocular suspension:
Intraocular Administration:
Dextenza Ophthalmic Insert
Intracanalicular Administration:
Otic Administration of Ophthalmic Solution:
Intravitreal Implant Administration
Pharmacologic doses of systemic corticosteroids (e.g. dexamethasone) administered for prolonged periods can result in physiological dependence due to hypothalamic-pituitary-adrenal (HPA) suppression. Exogenously administered corticosteroids exert a negative feedback effect on the pituitary, inhibiting the secretion of adrenocorticotropin (ACTH). This results in a decrease in ACTH-mediated synthesis of endogenous corticosteroids and androgens by the adrenal cortex. The severity of secondary adrenocortical insufficiency varies among individuals and is dependent on the dose, frequency, time of administration, and duration of therapy. Systemic administration of the drug on alternate days may help to alleviate this adverse effect. Patients with HPA suppression will require increased doses of corticosteroid therapy during periods of physiologic stress. Acute adrenal insufficiency and even death can occur with abrupt discontinuation of therapy. Discontinuation of prolonged oral corticosteroid therapy should be gradual since HPA suppression can last for up to 12 months following cessation of therapy. Patients may continue to need supplemental corticosteroid treatment during periods of physiologic stress or infectious conditions, even after the drug has been discontinued. A withdrawal syndrome unrelated to adrenocortical insufficiency can occur following sudden discontinuance of corticosteroid therapy. This syndrome includes symptoms such as appetite loss, malaise, lethargy, nauseousness, head pain/ache, joint pain, muscle pain, fever, exfoliative dermatitis, loss of weight, and hypotension. These effects are believed to be due to the sudden change in corticosteroid concentration rather than to low corticosteroid levels. Increased intracranial pressure with papilledema (i.e., pseudotumor cerebri) has also been reported with glucocorticoids usually after treatment withdrawal.[60760] [60761] [64165]
Prolonged systemic dexamethasone therapy can adversely affect the endocrine system, resulting in hypercorticism (Cushing syndrome including fat abnormalities such as buffalo hump and moon face), hypertrichosis or hirsutism, menstrual irregularity, or a decrease in carbohydrate or glucose tolerance.[60760] [60761] [64165]
Systemic corticosteroids are a common cause of drug-induced hyperglycemia. In the hospital setting, there is evidence that more than 50% of the patients receiving high-dose systemic steroids develop hyperglycemia, with many more having at least 1 episode of hyperglycemia or a mean blood glucose of 140 mg/dL or greater. Long-term use produces metabolic and endocrine effects that include insulin resistance that may lead to new diagnoses of diabetes mellitus (DM) in patients without a history of hyperglycemia or DM prior to corticosteroid use. Glucosuria (glycosuria) and aggravation of existing DM may also occur.[68700] [60760] [60761] [64165]
Endogenous glucocorticoids are responsible for protein metabolism; prolonged therapy with pharmaceutical glucocorticoids like dexamethasone can result in various musculoskeletal and joint manifestations, including myopathy (myalgia, muscle wasting, muscle weakness or myasthenia, and quadriplegia), arthralgia, tendon rupture, bone matrix atrophy (osteoporosis and osteopenia), bone fractures such as vertebral compression fractures or fractures of long bones, and avascular necrosis of femoral or humeral heads. These effects are more likely to occur in older or debilitated patients. Of note, abrupt cessation of corticosteroids can cause arthralgia and myalgia. Glucocorticoids interact with calcium metabolism at many sites, including: decreasing the synthesis by osteoblasts of the principal proteins of bone matrix, malabsorption of calcium in both the nephron and the gut, and reduction of sex hormone concentrations. Although all of these actions probably contribute to glucocorticoid-induced osteoporosis, the actions on osteoblasts are most important. Glucocorticoids do not modify vitamin D metabolism. Intra-articular injections of corticosteroids can cause Charcot-like arthropathy and post-injection flare. Atrophy at the site of injection has been reported following administration of soluble glucocorticoids.[24837] [60760] [60761] [64165] Because of retardation of bone growth, children receiving prolonged systemic corticosteroid therapy, like dexamethasone, may have growth inhibition. Growth inhibition has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients.[60760] [60761] [64165]
Adverse gastrointestinal (GI) effects associated with systemic corticosteroid (e.g., dexamethasone) administration include nausea and vomiting. Abdominal pain or distention, appetite stimulation, weight gain, pancreatitis, gastritis, hiccups, peptic ulcer with possible GI perforation and GI bleeding, perforation of the small and large bowel (particularly in patients with inflammatory bowel disease), and esophageal ulceration (ulcerative esophagitis) have also been reported.[60760] [60761] [64165] Although it was once believed that corticosteroids contributed to the development of peptic ulcer disease, in a review of 93 studies of corticosteroid use, the incidence of peptic ulcer disease was not found to be higher in steroid recipients compared to control groups. While most of these studies did not utilize endoscopy, it is unlikely that corticosteroids contribute to the development of peptic ulcer disease.[24362]
Corticosteroid therapy including dexamethasone can mask the symptoms of infection and should generally be avoided during an acute viral, fungal, or bacterial infection. Leukocytosis is a common physiologic effect of systemic corticosteroid therapy and may need to be differentiated from the leukocytosis that occurs with inflammatory or infectious processes.[30943] [65096] [65097] Immunosuppression from corticosteroids is most likely to occur in patients receiving high-dose (e.g., equivalent to 1 mg/kg or more of prednisone daily), systemic corticosteroid therapy for any period of time, particularly in conjunction with corticosteroid-sparing drugs (e.g., troleandomycin) and/or concomitant immunosuppressant agents; however, patients receiving moderate dosages of systemic corticosteroids for short periods or low dosages for prolonged periods may also be at risk. Corticosteroid-induced immunosuppression may result in the activation of latent viral (e.g., herpes) or bacterial (e.g., tuberculosis) infections and should not be used in patients with an active infection except when appropriate anti-infective therapy is instituted concomitantly. Patients receiving immunosuppressive doses of corticosteroids should be advised to avoid exposure to measles or varicella (chickenpox) and, if exposed to these diseases, to seek medical advice immediately. Monitoring systemic corticosteroid recipients for signs of opportunistic fungal infection is recommended, as cases of oropharyngeal candidiasis have been reported. Development of Kaposi's sarcoma has also been associated with prolonged administration of corticosteroids; discontinuation of the corticosteroid may result in clinical improvement.[60760] [60761] [64165] Bronchitis was noted in 5% of dexamethasone ophthalmic implant recipients during clinical trials and at an incidence higher than with placebo; secondary ophthalmic infection or exacerbation of infection has also been reported with other ophthalmic and intraocular dosage forms.[41921] [48640] [54348]
Various adverse dermatologic effects reported during systemic corticosteroid therapy include skin atrophy (thin fragile skin), increased sweating (hyperhidrosis), acne vulgaris, striae, acneiform rash, alopecia, xerosis, perineal pain and irritation, purpura, rash (unspecified), telangiectasia, facial erythema, petechiae, ecchymosis or easy bruising, and suppression of reactions to skin tests. An increased susceptibility to skin ulcer may occur in patients with impaired circulation. Hypersensitivity reactions may manifest as allergic dermatitis, urticaria, anaphylactoid reactions, and/or angioedema. Burning or tingling in the perineal area may occur following IV injection of corticosteroids. Parenteral corticosteroid therapy has also produced skin hypopigmentation, skin hyperpigmentation, scarring, and other types of injection site reaction (e.g., induration, delayed pain or soreness, subcutaneous and cutaneous atrophy, and sterile abscesses).[60760] [60761] [64165]
In general, systemic corticosteroids like dexamethasone can lead to impaired wound healing.[60760] [60761] [64165]
Prolonged administration of systemic dexamethasone can result in edema and fluid retention due to sodium retention; electrolyte disturbances (hypokalemia, hypokalemic metabolic alkalosis, hypernatremia, hypocalcemia); and hypertension.[60760] [60761] [64165] In a review of 93 studies of corticosteroid use, hypertension was found to develop 4 times as often in steroid recipients compared to control groups.[24362] In another study, an increased risk of heart failure was observed for medium-dose glucocorticoid use as compared with nonuse. At the beginning of the study, patients were at least 40 years of age and had not been hospitalized for cardiovascular disease. Medium exposure was defined as less than 7.5 mg daily of prednisolone or the equivalent given orally, rectally, or parenterally.[30697] Increased blood pressure was noted in 13% of dexamethasone ophthalmic insert recipients for one of the products during clinical trials.[41921]
Adverse neurologic effects have been reported during prolonged systemic dexamethasone administration and include insomnia, vertigo or dizziness, restlessness, amnesia and memory impairment, increased motor activity, impaired cognition, paresthesias, ischemic peripheral neuropathy, seizures, neuritis, and EEG changes. Mental disturbances, including depression, anxiety, euphoria, personality changes, emotional lability, delirium, dementia, hallucinations, irritability, mania, mood swings, schizophrenic reactions, withdrawn behavior, and psychosis have also been reported; emotional lability and psychotic problems can be exacerbated by corticosteroid therapy. Headache may be a sign of elevated intracranial pressure.[60760] [60761] [64165] Arachnoiditis, meningitis, paresis, paraplegia, and sensory disturbances have occurred after intrathecal administration. Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids. Specific events reported include, but are not limited to, spinal cord infarction, paraplegia, quadriplegia, cortical blindness, and stroke.[60760] Headache was noted in 1% to 4% of dexamethasone ophthalmic insert/implant recipients during clinical trials, at incidences higher than with placebo.[41921] [63796]
Dexamethasone can cause increased intraocular pressure or ocular hypertension, the magnitude of which depends on the formulation used, indication for use, and the frequency and duration of dosing. Ocular hypertension can occur after 1 to 6 weeks of topical ophthalmic therapy, and it is usually reversible upon discontinuance of the drug. Use of the intravitreal implant has resulted in ocular hypertension (glaucoma) in 5% of patients in clinical trials and increased ocular pressure (IOP) occurred in 25% to 35% of patients receiving the intravitreal implant. In retinal vein occlusion (RVO) and uveitis trials, IOP peaked at 60 days, returning to baseline by day 180. During the initial treatment period with the intravitreal implant for RVO and uveitis, 1% of patients required laser or surgical procedures to manage elevated IOP. In a 2-year observational study with the intravitreal implant, among patients who received more than 2 injections, increased IOP was reported in 24% (n = 68) patients. Frequently check IOP during receipt of ophthalmic preparations of dexamethasone. In diseases that cause thinning of the cornea or sclera, topical ocular steroids have been known to cause perforation. Intravitreal injections have also been associated with endophthalmitis, ocular inflammation, and retinal detachment. In clinical trials, the use of the intravitreal implant has resulted in ocular hemorrhage (conjunctival hemorrhage) (22% to 23%), ocular pain (8%), conjunctival hyperemia (7%), conjunctivitis (6%), vitreous floaters (5%), conjunctival edema (5%), xerophthalmia (5%), vitreous opacities (3%), retinal aneurysm (3%), foreign body sensation (2%), corneal erosion (2%), keratitis (2%), anterior chamber inflammation (2%), retinal tear (2%), eyelid ptosis (2%), vitreous hemorrhage (ocular hemorrhage, 6%), and vitreous detachment (2% to 4%). Postmarketing, complication of device insertion resulting in ocular tissue injury including sclera, subconjunctiva, lens and retina (implant misplacement), device dislocation with or without corneal edema, and ocular hypotonia (associated with vitreous leakage due to injection) were noted. Patients with an absent or torn posterior capsule of the lens are at increased risk of migration of the intravitreal implant into the anterior chamber.[41921] The most common ocular adverse reactions that occurred in patients treated with dexamethasone ophthalmic insert for ocular inflammation and pain following ophthalmic surgery were: anterior chamber inflammation including iritis and iridocyclitis (10%), increased intraocular pressure (6%), reduced visual acuity (2%), ocular pain (1%), cystoid macular edema (1%), corneal edema (1%), and conjunctival hyperemia (1%). The most common ocular adverse reactions that occurred in patients treated with dexamethasone ophthalmic insert for itching associated with allergic conjunctivitis were: increased intraocular pressure (3%), increased lacrimation (1%), ocular discharge (1%), and reduced visual acuity (1%).[63796] Ocular irritation including transient stinging, burning, or tearing and keratoconjunctivitis may occur during use of ophthalmic dexamethasone. Allergic reactions have also been reported; ocular pruritus can occur. Ocular discomfort (10%) and eye irritation (1%) were the most frequently reported adverse reactions in clinical studies with dexamethasone ophthalmic suspension. All other adverse reactions from these studies occurred with a frequency less than 1% including keratitis, conjunctivitis, dry eye (xerophthalmia), photophobia, blurred vision, ocular pruritus, foreign body sensation, increased lacrimation, abnormal ocular sensation, eyelid margin crusting, and ocular hyperemia. Postmarketing adverse reactions with dexamethasone topical ophthalmic suspension use include corneal erosion, dizziness, ocular pain, eyelid ptosis, headache, hypersensitivity reactions, and mydriasis.[61633] In patients receiving dexamethasone intraocular suspension for injection, the most common adverse reactions occurring in 5% to 15% of patients included intraocular pressure or ocular hypertension, corneal edema and iritis. Other ocular adverse reactions occurring in 1% to 5% of patients included corneal endothelial cell loss, blepharitis, ocular pain, cystoid macular edema, xerophthalmia, ocular inflammation, posterior capsule opacification, blurred vision, reduced visual acuity, vitreous floaters, foreign body sensation, photophobia, and vitreous detachment.[48640] Prolonged use of dexamethasone therapy by any route can result in ocular nerve damage including optic neuritis and visual defects. Temporary or permanent visual impairment, including blurred vision and blindness, has been reported with glucocorticoid administration by several routes of administration including intranasal and ophthalmic administration. Other ocular adverse reactions resulting from systemic corticosteroid therapy can include corneal perforation, exophthalmos, slowing of corneal wound healing, increased intraocular pressure, glaucoma with possible damage to the optic nerves, blurred vision, or retinopathy.[60760] [60761] [64165] Consider referring patients who develop ocular symptoms or use systemic corticosteroid-containing products for more than 6 weeks to an ophthalmologist for evaluation.[41921] [48640] [60760] [60761] [61633] [64165] Dexamethasone (by any route) can reduce host resistance to infection. Secondary fungal and viral infections of the eye (ocular infection) can be masked or exacerbated by corticosteroid therapy. Investigate the possibility of fungal infection if patients have persistent corneal ulceration. Prolonged use of dexamethasone therapy by any route has resulted in posterior subcapsular cataracts.[48640] [60760] [60761] [64165] The mechanism of corticosteroid-induced cataract formation is uncertain but may involve disruption of sodium-potassium pumps in the lens epithelium leading to accumulation of water in lens fibers and agglutination of lens proteins.[24813] The incidence of cataracts with initial use of the intravitreal implant in clinical trials of patients with RVO or uveitis was 5% within the first 6 months; however, the overall incidence after a second intravitreal implant injection was higher after 1 year. In diabetic macular edema (DME) trials, the incidence of cataract development in patients who had a phakic study eye was higher in the dexamethasone group (68%) compared with sham (21%). Among these patients, 61% of dexamethasone subjects vs. 8% of sham-controlled subjects underwent cataract surgery. In a 2-year observational study, among patients who received more than 2 injections, the most frequent adverse reaction was cataract 54% (n = 96 out of 178 phakic eyes at baseline).[41921]
Hypercholesterolemia, atherosclerosis, fat embolism, palpitations, sinus tachycardia, bradycardia, syncope, vasculitis, necrotizing angiitis, thrombosis, thromboembolism, and phlebitis, specifically, thrombophlebitis, have been associated with systemic corticosteroid therapy such as dexamethasone. Systemic glucocorticoid use appears to increase the risk of cardiovascular events such as myocardial infarction, left ventricular rupture (in persons who recently experienced a myocardial infarction), angina, angioplasty, coronary revascularization, stroke, transient ischemic attack, cardiomegaly, arrhythmia exacerbation and ECG changes, hypertrophic cardiomyopathy (in premature infants), congestive heart failure and pulmonary edema, cardiac arrest or cardiovascular death.[60760] [60761] [64165] As determined from observational data, the rate of cardiovascular events was 17 per 1,000 person-years among 82,202 non-users of glucocorticoids. In contrast, the rate was 23.9 per 1,000 person-years among 68,781 glucocorticoid users. Furthermore, the rate of cardiovascular events was 76.5 per 1,000 person-years for high exposure patients. After adjustment for known covariates by multivariate analysis, high-dose glucocorticoid use was associated with a 2.56-fold increased risk of cardiovascular events as compared with nonuse. At the beginning of the study, patients were at least 40 years of age and had not been hospitalized for cardiovascular disease. High glucocorticoid exposure was defined as at least 7.5 mg daily of prednisolone (or equivalent) given orally, rectally, or parenterally whereas medium exposure was defined as less than the above dosage by any of the 3 routes. Low-dose exposure was defined as inhaled, topical, or nasal usage only.[30697]
Cases of elevated hepatic enzymes (usually reversible upon discontinuation) and hepatomegaly have been associated with corticosteroid receipt such as dexamethasone.[60760] [60761] [64165]
The coadministration of certain medications may lead to harm and require avoidance or therapy modification; review all drug interactions prior to concomitant use of other medications.
This medication is contraindicated in patients with a history of hypersensitivity to it or any of its components.
The use of systemic corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes.[60760][60761][64165]
The use of ophthalmic dexamethasone formulations (including ocular implant, ophthalmic insert, and opthalmic drops), is contraindicated in herpes simplex virus epithelial keratitis, the acute infectious stages of vaccinia (smallpox), varicella, and many other viral ocular infection diseases of the cornea and conjunctiva, mycobacterial infection of the eye, and fungal diseases of ocular or auricular structures.[48640][41921][63796][49533][54348]
Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (e.g., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead the development of osteopenia and osteoporosis at any age. Special consideration should be given to people at increased risk for osteoporosis (e.g., postmenopausal females) before initiating corticosteroid therapy.[51792] [60760] [60761] [64165]
Systemic corticosteroids, including dexamethasone, may cause dose-dependent immunosuppression and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids may mask symptoms and increase the risk of severe or fatal infectious complications, requiring close monitoring and potential dose adjustment or withdrawal. Systemic dexamethasone and other corticosteroids may cause severe or fatal infections by reactivating or worsening TB, hepatitis B, amebiasis, Strongyloides, varicella, measles, herpes simplex, and other viral or herpes infections. Avoid using corticosteroids in individuals with cerebral malaria. Screen for hepatitis B before prolonged immunosuppressive therapy; consult specialists and consider antiviral prophylaxis if infection is present. Rule out latent amebiasis in individuals with tropical exposure or unexplained diarrhea. Use caution in individuals with known or suspected Strongyloides infection; prophylactic therapy may be indicated. It is contraindicated in individuals with systemic fungal infection. Avoid use in systemic fungal infections unless essential; discontinue or reduce corticosteroid if fungal infection develops. Corticosteroids should be used with caution, if at all, in individuals with ocular herpes simplex. Avoid exposing nonimmune corticosteroid-treated individuals to varicella or measles. If exposure occurs, prophylaxis with varicella zoster immune globulin (VZIG) for varicella or immunoglobulin (IG) for measles may be needed. If infection develops, consider antiviral treatment. Do not inject intra-articularly, intra-bursally, or intratendinously in areas of acute infection.[65868] [60760] [60761] [64165]
Corticosteroid therapy, including systemic dexamethasone therapy, has been associated with left ventricular free-wall rupture in individuals with recent myocardial infarction, and should therefore be used cautiously in these individuals. As sodium retention with resultant edema and potassium loss may occur in individuals receiving systemic corticosteroids, these agents should be used with caution in individuals with congestive heart failure, hypertension, renal impairment, or renal failure.[60760] [60761] [64165]
Systemic corticosteroids, such as dexamethasone, may decrease glucose tolerance, produce hyperglycemia, and aggravate or precipitate diabetes mellitus. This may especially occur in individuals predisposed to diabetes mellitus. When corticosteroid therapy is necessary for individuals with diabetes mellitus, changes in insulin, oral antidiabetic agent dosage, and/or diet may be required.[60760] [60761] [64165]
An acute myopathy has been observed with the use of high doses of systemic corticosteroids, most often occurring in individuals with neuromuscular transmission, including myasthenia gravis, or in individuals receiving concomitant therapy with neuromuscular blocking drugs. This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.[60760] [60761] [64165]
Systemic corticosteroid use may be associated with neuro-psychiatric effects ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychosis. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids, such as in individuals with a mood disorder or psychotic disorder. Inform individuals or caregivers of the potential for mood and behavioral changes with systemic dexamethasone treatment and encourage them to seek medical attention if psychiatric symptoms develop, especially if depressed mood (depression) or suicidal ideation is suspected.[60760] [60761] [64165]
Metabolic clearance of corticosteroids is decreased in hypothyroidism and increased in hyperthyroidism. Changes in thyroid disease status of an individual may necessitate an adjustment in systemic dexamethasone dosage.[60760] [60761] [64165]
Systemic corticosteroids should be used with caution in individuals with active or latent peptic ulcer disease, diverticulitis, fresh intestinal anastomosis, and nonspecific ulcerative colitis, since steroids may increase the risk of a gastrointestinal (GI) perforation. Signs of peritoneal irritation following GI perforation in individuals receiving corticosteroids may be minimal or absent. Corticosteroids should not be used in individuals where there is a possibility of impending GI perforation, GI abscess, or pyogenic infection. There is an enhanced effect due to decreased metabolism of systemic corticosteroids in individuals with hepatic cirrhosis.[60760] [60761] [64165]
There are no adequate, well-controlled studies for the use of dexamethasone in pregnant women; therefore, the manufacturers recommend that the drug be used during pregnancy only if the potential benefit to the mother outweighs the potential risk to the fetus. For COVID-19, the National Institutes of Health (NIH) recommends use of the drug in pregnant patients, if indicated, as the potential benefit of decreased maternal mortality justifies the low risk of fetal adverse effects with the short course of therapy.[65314] Corticosteroids have been shown to be teratogenic in many species when given in systemic doses equivalent to the human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring.[60760] [60761] [64165] In addition, dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application in multiples of the therapeutic dose.[61633] Topical ocular administration of dexamethasone to pregnant mice and rabbits during organogenesis produced embryofetal lethality, cleft palate and multiple visceral malformations.[41921] [63796] Topical and otic corticosteroids should not be used in large amounts, on large areas, or for prolonged periods of time in pregnant women. Dexamethasone injections have been used medically later in pregnancy to induce fetal lung maturation in patients at risk for pre-term delivery; use is for select circumstances and for a limited duration of time.[33038] [33039] [33040] An infant who is born to a woman receiving large doses of systemic corticosteroids during pregnancy should be monitored for signs of adrenal insufficiency, and appropriate therapy should be initiated, if necessary.
The dexamethasone intravitreal implant is contraindicated in individuals with glaucoma who have cup to disc ratio more than 0.8. Dexamethasone intravitreal implant is also contraindicated in individuals who have a tear or a rupture of posterior ocular lens capsule; these individuals with an absent or torn posterior capsule of the lens are at increased risk of migration of the intravitreal implant into the anterior chamber.[41921]
Systemic use of dexamethasone has not been studied during breast-feeding; corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution is warranted, and some manufacturers recommend discontinuing breast-feeding if systemic dexamethasone treatment is needed.[60760] [60761] [64165] However, experts generally consider inhaled corticosteroids and oral corticosteroids (e.g., prednisone and prednisolone), acceptable to use during breast-feeding.[33723] [33724] [31822] There is no information regarding dexamethasone effects on breastfed infants or milk production or its presence in human milk following placement of the intravitreal implant or intracanalicular insert to inform risk to an infant during lactation.[41921] [63796] However, the systemic concentration of dexamethasone following administration of the intracanalicular insert is low.[63796] It is not known whether topical ophthalmic administration of dexamethasone could result in sufficient systemic absorption to produce detectable quantities in breast milk.[61633] [49533] For COVID-19, the National Institutes of Health (NIH) recommends dexamethasone be offered to lactating mothers who qualify for therapy without interruption of breast-feeding.[65314] Consider the benefits of breast-feeding, the risk of potential infant drug exposure, and the risk of an untreated or inadequately treated condition.
Use systemic corticosteroids with caution in the geriatric individual. Geriatric and debilitated adults are especially susceptible to corticosteroid-induced decreases in bone mineral density and resultant fractures. Detrimental effects on bone metabolism, such as osteoporosis, are a risk with chronic, systemically-administered corticosteroids.[60760] [60761] [64165] According to the Beers Criteria, systemic corticosteroids are considered potentially inappropriate medications (PIMs) for geriatric adults with delirium or at high risk for delirium; avoid in these patient populations due to the possibility of new-onset delirium or exacerbation of the current condition. Oral and parenteral corticosteroids may be required for conditions, such as exacerbation of chronic obstructive pulmonary disease (COPD), but should be prescribed in the lowest effective dose and for the shortest possible duration.[63923]
The routine use of high-dose (greater than 0.5 mg/kg/day) dexamethasone for either the prevention or treatment of chronic lung disease in premature neonates is not recommended due to a lack of survival benefit and concern about long-term adverse outcomes, particularly increased rates of cerebral palsy. Studies utilizing lower doses of dexamethasone (less than 0.2 mg/kg/day) have not reported increased rates of adverse neurodevelopmental effects; however, due to the small number of participants included in these studies, the AAP states that there is insufficient evidence to recommend the use of low-dose dexamethasone and further study is warranted.[54338] In a geographical cohort study of 148 extremely premature pediatric patients (born less than 28 weeks gestation), 55 (27%) received postnatal dexamethasone (mean cumulative dose 7.7 mg/kg) during the neonatal period. Participants receiving dexamethasone had smaller total brain tissue volume (mean difference -3.6%, p value = 0.04) and smaller white matter, thalami, and basal ganglia volumes (p is less than 0.05 for all) when compared with participants who did not receive postnatal dexamethasone. There was also a trend of smaller total brain and white matter volumes with an increased dose of postnatal dexamethasone.[56910] Dexamethasone injectable formulations may contain benzyl alcohol. Serious and fatal adverse reactions including "gasping syndrome" can occur in neonates, premature neonates, and low birth weight infants treated with benzyl-alcohol preserved drugs. The "gasping syndrome" is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions can occur is not known.[72735]
Dexamethasone ophthalmic solutions are sometimes used off-label in the ear for otic conditions. Otic dexamethasone use is contraindicated for use in individuals with tympanic membrane perforation.[54348]
Glucocorticoids are naturally occurring hormones that prevent or suppress inflammation and immune responses when administered at pharmacological doses. At the molecular level, unbound glucocorticoids readily cross cell membranes and bind with high affinity to specific cytoplasmic receptors. This binding induces a response by modifying transcription and, ultimately, protein synthesis to achieve the steroid's intended action. Such actions can include: inhibition of leukocyte infiltration at the site of inflammation, interference in the function of mediators of the inflammatory response, and suppression of humoral immune responses. Some of the net effects include reduction in edema or scar tissue and a general suppression in an immune response. The degree of clinical effect is normally related to the dose administered. The anti-inflammatory actions of corticosteroids are thought to involve phospholipase A2 inhibitory proteins, collectively called lipocortins. Lipocortins, in turn, control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of the precursor molecule arachidonic acid. Likewise, the numerous adverse effects related to corticosteroid use usually depend on the dose administered and the duration of therapy.[30943][50600]
Revision Date: 10/06/2025, 03:26:25 PMDexamethasone is administered via oral, intravenous, intramuscular, intraarticular, intravitreal, ophthalmic, and otic routes. Certain dosage forms, like inhalational products, have been removed from marketing. Circulating drug binds weakly to plasma proteins, with only the unbound portion of a dose being active. Systemic dexamethasone is quickly distributed into the kidneys, intestines, skin, liver, and muscle. Corticosteroids distribute into breast milk and cross the placenta. Systemic dexamethasone is metabolized by the liver to inactive metabolites. These inactive metabolites, as well as a small portion of unchanged drug, are excreted in the urine. The plasma elimination half-life of dexamethasone is approximately 1.8 to 3.5 hours whereas the biological half-life is 36 to 54 hours.[34477]
Affected cytochrome P450 (CYP450) isoenzymes and drug transporters: CYP3A4, P-glycoprotein (P-gp)
Dexamethasone is a weak inducer of CYP3A4 and is a substrate for both P-glycoprotein (P-gp) and CYP3A4.[34477][69153]
Dexamethasone is rapidly and well absorbed after oral administration.[38183] In adults, bioavailability has been reported to be in the range of approximately 60% to 100%, with no significant differences between the elixir and tablet formulations.[54046][54366] Peak concentrations occur 1 to 2 hours after oral administration.[54046] However, 1 study of 13 patients (aged 14 to 28 years) with congenital adrenal hyperplasia reported a mean time to peak concentrations for oral dexamethasone of 45 minutes (range 30 to 120 minutes).[54352]
Peak concentrations were reached approximately 60 minutes after single-dose administration of IV dexamethasone in neonates.[54368]
The onset and duration of action of dexamethasone injection ranges from 2 days to 3 weeks and is dependent on whether the drug is administered by intra-articular or IM injection and by the extent of the local blood supply.
Intra-articular Route
The onset and duration of action of dexamethasone injection ranges from 2 days to 3 weeks and is dependent on whether the drug is administered by intra-articular or IM injection and by the extent of the local blood supply.
Ophthalmic Route
Following ophthalmic administration, dexamethasone is absorbed through the aqueous humor and distribute into the local tissues, with only minimal systemic absorption occurring. Ophthalmic doses are metabolized locally.
Intravitreal Implant Route
After the insertion of the dexamethasone intravitreal implant (0.35 mg or 0.7 mg) in 21 patients, plasma concentrations were obtained on days 1, 7, 30, 60, and 90. Overall, the majority of dexamethasone plasma concentration measurements were below the lower limit of quantitation (LLOQ = 50 pg/mL). Ten of the 73 samples in the patients receiving the 0.7 mg dose and 2 of the 42 samples in the patients receiving the 0.35 mg dose were above the LLOQ (range, 52 to 94 pg/mL). The highest plasma concentration (94 pg/mL) was observed in one patient who had received the 0.7 mg dose. Age, body weight, and gender did not affect the plasma dexamethasone concentrations. In vitro metabolism studies of the intravitreal implant showed no metabolites.[41921]
Intraocular Route
Systemic exposure to dexamethasone was evaluated in a subgroup of patients enrolled in 2 studies (n = 25 for the first study and n = 13 for the second study). The patients received a single intraocular injection of dexamethasone containing 342 mcg or 517 mcg of dexamethasone at the end of cataract surgery and blood samples were collected prior to surgery and at the several time points post-surgery between Day 1 and up to Day 30. In the first study, the dexamethasone plasma concentrations on post-surgery Day 1 ranged from 0.09 to 0.86 ng/mL and from 0.07 to 1.16 ng/mL following administration of dexamethasone 342 mcg and 517 mcg, respectively. In the second study, dexamethasone plasma concentrations on post-surgery Day 1 ranged from 0.349 to 2.79 ng/mL following administration of dexamethasone 517 mcg. In both the studies, dexamethasone plasma concentrations declined over time and very few patients had quantifiable dexamethasone plasma concentrations at the final time point of sampling (Day 15 or Day 30).[48640]
Intracanalicular Route
Systemic exposure to dexamthasone was evaluated in 16 healthy volunteers. Plasma samples were obtained prior to and at several time points on Days 1 to 29. Dexamethasone plasma concentrations were detectable (above 50 pg/mL, the lower limit of quantification of the assay) in 11% of samples (21 of 189), and ranged from 0.05 ng/mL to 0.81 ng/mL.[63796]
In adult patients with chronic liver disease, dexamethasone clearance is reduced and the elimination half-life is prolonged. Pharmacokinetic data are unavailable in pediatric patients with hepatic impairment.[54046]
In adult patients with renal impairment, dexamethasone clearance is increased and the elimination half-life is shorter. This is due to decreased protein binding of dexamethasone to albumin in uremic patients. Pharmacokinetic data are unavailable in pediatric patients with renal impairment.[54046]
Infants, Children, and Adolescents
Pharmacokinetics of dexamethasone in pediatric patients are similar to adults. In a pharmacokinetic study in 12 pediatric patients (4 months to 16 years) who received IV dexamethasone (0.1 to 0.3 mg/kg/dose), the mean elimination half-life of dexamethasone was 4.34 hours (range 2.33 to 9.54 hours), which is similar to that reported in adults. The mean volume of distribution (Vd) was 2.07 L/kg (range 0.48 to 8.99 L/kg).[54361] Another study that included adolescents and adults (14 to 28 years) reported a mean elimination half-life of 3.53 hours (range 2.18 to 4.5 hours) after dexamethasone administration.[54352]
Neonates
Clearance of dexamethasone in neonates is a function of gestational age (GA) with premature neonates having a slower clearance. In a pharmacokinetic study in 9 neonates (mean GA 27.3 weeks [range 25 to 30 weeks]; mean postnatal age 21.8 days), mean clearance was 1.69 mL/kg/minute in neonates with a GA less than 27 weeks compared with 7.57 mL/kg/minute in neonates with a GA more than 27 weeks. Corresponding elimination half-life values were 10.2 and 4.9 hours, respectively. The mean Vd was 1.78 L/kg, which was also correlated with GA (1.26 vs 2.19 L/kg for neonates with GA less than 27 weeks and more than 27 weeks, respectively). The mean Vd was higher than what has been reported in adults (0.77 L/kg).[54358] Another study in 7 extremely low birth weight neonates (mean GA 25.6 weeks; mean birthweight 735 g) found similar results after administration of single-dose IV dexamethasone. In this study, mean values for clearance, Vd, and elimination half-life were 2.4 mL/kg/minute, 1.9 L/kg, and 9.26 hours, respectively.[54368]
There are no adequate, well-controlled studies for the use of dexamethasone in pregnant women; therefore, the manufacturers recommend that the drug be used during pregnancy only if the potential benefit to the mother outweighs the potential risk to the fetus. For COVID-19, the National Institutes of Health (NIH) recommends use of the drug in pregnant patients, if indicated, as the potential benefit of decreased maternal mortality justifies the low risk of fetal adverse effects with the short course of therapy.[65314] Corticosteroids have been shown to be teratogenic in many species when given in systemic doses equivalent to the human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring.[60760] [60761] [64165] In addition, dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application in multiples of the therapeutic dose.[61633] Topical ocular administration of dexamethasone to pregnant mice and rabbits during organogenesis produced embryofetal lethality, cleft palate and multiple visceral malformations.[41921] [63796] Topical and otic corticosteroids should not be used in large amounts, on large areas, or for prolonged periods of time in pregnant women. Dexamethasone injections have been used medically later in pregnancy to induce fetal lung maturation in patients at risk for pre-term delivery; use is for select circumstances and for a limited duration of time.[33038] [33039] [33040] An infant who is born to a woman receiving large doses of systemic corticosteroids during pregnancy should be monitored for signs of adrenal insufficiency, and appropriate therapy should be initiated, if necessary.
Systemic use of dexamethasone has not been studied during breast-feeding; corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution is warranted, and some manufacturers recommend discontinuing breast-feeding if systemic dexamethasone treatment is needed.[60760] [60761] [64165] However, experts generally consider inhaled corticosteroids and oral corticosteroids (e.g., prednisone and prednisolone), acceptable to use during breast-feeding.[33723] [33724] [31822] There is no information regarding dexamethasone effects on breastfed infants or milk production or its presence in human milk following placement of the intravitreal implant or intracanalicular insert to inform risk to an infant during lactation.[41921] [63796] However, the systemic concentration of dexamethasone following administration of the intracanalicular insert is low.[63796] It is not known whether topical ophthalmic administration of dexamethasone could result in sufficient systemic absorption to produce detectable quantities in breast milk.[61633] [49533] For COVID-19, the National Institutes of Health (NIH) recommends dexamethasone be offered to lactating mothers who qualify for therapy without interruption of breast-feeding.[65314] Consider the benefits of breast-feeding, the risk of potential infant drug exposure, and the risk of an untreated or inadequately treated condition.
Cookies are used by this site. To decline or learn more, visit our cookie notice.
Copyright © 2025 Elsevier, its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.