ThisiscontentfromElsevier'sDrugInformation
Prednisone
Learn more about Elsevier's Drug Information today! Get the drug data and decision support you need, including TRUE Daily Updates™ including every day including weekends and holidays.
General dosing information for systemic therapy
Estimated equivalent systemic Glucocorticoid dosages. These are general approximations and may not apply to all diseases or routes of administration.[51324]
Cortisone-25 mg
Hydrocortisone-20 mg
Prednisolone-5 mg
Prednisone-5 mg
Methylprednisolone-4 mg
Triamcinolone-4 mg
Dexamethasone-0.75 mg
Betamethasone-0.75 mg
General Instructions for Delayed-release prednisone tablets (e.g., Rayos)
Dosage for Rayos is in the range of 5 mg/day to 60 mg/day PO once daily. When deciding the administration time for the delayed-release tablets, consider the pharmacokinetics and the disease or condition being treated. Prednisone is released from the tablet beginning approximately 4 hours after intake of the first dose.[51324]
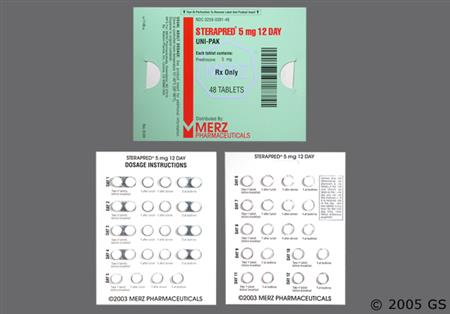
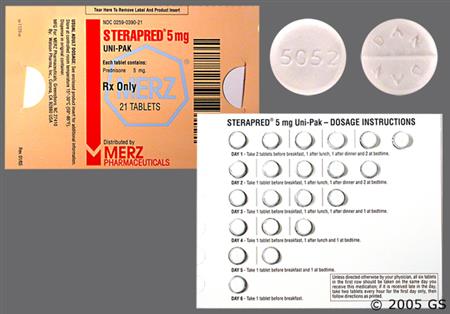
General Instructions for prednisone dose packs (e.g., Sterapred Uni-Pak, Sterapred DS Uni-Pak, and generic equivalents)
NOTE: While packages are typically labeled with the following instructions, the proper dosage tapers should be determined by decreasing the initial dosage in small decrements at appropriate time intervals. Dosage adjustments may be necessary for changes in clinical status (remissions or exacerbations in the disease process), the patient's individual drug responsiveness, and patient exposure to stressful situations; in the latter situation, it may be necessary to increase dosage for a period of time. Constant monitoring is needed in regard to drug dosage.[41659]
Adult Oral dosage (Sterapred 5 mg tablets or Sterapred-DS 10 mg tablets, 21-tablet dose pack):
Day 1: 2 tablets PO before breakfast, 1 tablet PO after lunch, 1 tablet PO after supper, and 2 tablets PO at bedtime.
Day 2: 1 tablet PO before breakfast. 1 tablet PO after lunch, 1 tablet PO after supper, and 2 tablets PO at bedtime.
Day 3: 1 tablet PO before breakfast, 1 tablet PO after lunch, 1 tablet PO after supper, and 1 tablet PO at bedtime.
Day 4: 1 tablet PO before breakfast, 1 tablet PO after lunch, and 1 tablet PO at bedtime.
Day 5: 1 tablet PO before breakfast, and 1 tablet PO at bedtime.
Day 6: 1 tablet PO before breakfast.
Adult Oral dosage (Sterapred 5 mg tablets or Sterapred-DS 10 mg tablets, 48-tablet dose pack):
Day 1 through 4: 2 tablets PO before breakfast, 1 tablet PO after lunch, 1 tablet PO after supper, and 2 tablets PO at bedtime.
Day 5 through 8: 1 tablet PO before breakfast. 1 tablet PO after lunch, 1 tablet PO after supper, and 1 tablet PO at bedtime.
Day 9 through 12: 1 tablet PO before breakfast, and 1 tablet PO at bedtime.
40 mg/day PO divided once or twice daily for up to 10 days or until hospital discharge, whichever comes first.[65876] [65314] The World Health Organization strongly recommends the use of systemic corticosteroids in people with severe or critical COVID-19.[65876] The National Institutes of Health (NIH) COVID-19 treatment guidelines recommend prednisone as an alternative corticosteroid for hospitalized individuals who require supplemental oxygen, including those on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). The NIH advises clinicians to review the individual's medical history and assess the potential risks and benefits before starting prednisone.[65314]
5 to 60 mg/day PO, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
1.5 to 3.5 mg/m2/day PO in 2 divided doses.[70941] Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324] Hydrocortisone is the preferred glucocorticoid until final height is reached; however, prednisone may be an acceptable alternative with close monitoring.[54155] [68698] [68699]
4 to 7.5 mg/day PO in 2 divided doses, or 1 to 2.5 mg/day PO in 2 divided doses when used in combination with hydrocortisone.[54155] [68699] [71840] The FDA-approved dosage is 5 to 60 mg/day PO, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
2 to 4 mg/m2/day PO in 2 divided doses.[54490] Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324] Hydrocortisone is the preferred glucocorticoid until final height is reached; however, prednisone may be an acceptable alternative with close monitoring.[54155] [68698] [68699]
1 to 4 mg/day PO in 1 or 2 divided doses.[71840] The FDA-approved dosage is 5 to 60 mg/day PO, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
Titrate to response. The usual range is 5 mg to 30 mg PO once daily. Renal transplant guidelines recommend a calcineurin inhibitor (CNI) such as tacrolimus and an antiproliferative agent such as mycophenolate plus or minus corticosteroids for initial prophylaxis. In patients at low immunologic risk who receive induction therapy, corticosteroid discontinuation during first week after transplantation is suggested. Some evidence exists that steroids may be safely stopped in most patients after 3 to 12 months on combination therapy with a CNI and mycophenolate. Data suggest that the risk of steroid withdrawal depends on the use of concomitant immunosuppressives, immunological risk, ethnicity, and time after transplantation.[51730] [51731]
1 mg/kg/dose PO once daily for 2 weeks, followed by an extended taper.[55037]
1 mg/kg/dose PO once daily for 2 weeks, followed by an extended taper.[55037]
40 mg/m2 to 50 mg/m2 PO once daily indefinitely.
Multiple dosage regimens have been studied. Initial dosage may vary from 5 mg/day to 60 mg/day PO. Dosage requirements are variable though and should be individualized based on the response of the patient and tolerance to treatment.[50175] NOTE: Prednisone is approved for the palliative treatment of CLL; however, all components of combination regimens may not have been evaluated by the FDA for the treatment of CLL.
30 mg/m2 PO daily for 5 days in combination with cladribine 0.12 mg/kg/day IV over 2 hours for 5 days repeated every 28 days for up to 6 cycles has been studied in a randomized trial.[50168] NOTE: Prednisone is approved for the palliative treatment; however, all components of combination regimens may not have been evaluated by the FDA for the treatment of CLL.
80 mg PO once daily on Day 1, Day 2, Day 3, Day 4, and Day 5 in combination with chlorambucil 30 mg/m2 PO on Day 1 repeated every 2 weeks for up to 18 months and maximum response was evaluated in a randomized study.[50813] Alternatively, prednisone 30 mg/m2 PO daily for 7 days plus chlorambucil 12 mg/m2 PO daily for 7 days repeated every 28 days for up to 6 courses was used in another randomized study.[50168] NOTE: Prednisone is approved for the palliative treatment of CLL; however, all components of combination regimens may not have been evaluated by the FDA for the treatment of CLL.
50 mg/day to 100 mg/day PO for 3 to 5 days is usually effective for hypercalcemia due to hematologic cancers, lower doses may be effective for some tumors.[23969]
2 mg/kg orally daily for 4 days plus melphalan 0.25 mg/kg orally daily for 4 days repeated every 6 weeks has been studied.[49722] Treatment cycles may be repeated when the granulocyte and platelet counts returned to normal. Response may be gradual over several months.[44928]
The optimal dosage of melphalan and prednisone plus thalidomide has not been clearly established and dosages have varied in randomized controlled trials.[33521] [49716] [49717] [49718] [49719] [49720] [49721] In one study, previously untreated patients between 65 and 75 years of age received melphalan (0.25 mg/kg PO daily) for 4 days and prednisone 2 mg/kg PO once daily for 4 days, cycles were repeated every 6 weeks for 12 cycles plus thalidomide (200 mg/day PO for 2 to 4 weeks escalated up to a maximum dose of 400 mg/day PO if no severe adverse events; most patients received thalidomide 200 mg/day or less). Thalidomide was stopped after day 4 of the last cycle.[33521] In another study, patients aged 75 years and older received melphalan (0.2 mg/kg PO daily) for 4 days and prednisone 2 mg/kg PO once daily for 4 days and repeated every 6 weeks for 12 cycles plus thalidomide 100 mg/day PO at bedtime.[49716]
60 mg/m2 orally daily on days 1, 2, 3, and 4 and melphalan 9 mg/m2 orally daily on days 1, 2, 3, and 4 plus bortezomib repeated every 6 weeks for 9 cycles. In cycles 1 through 4, bortezomib 1.3 mg/m2 IV or subcutanously is given on days 1, 4, 8, and 11 followed by a 10-day rest period (days 12 through 21) and again on days 22, 25, 29, and 32 followed by a 10-day rest period (days 33 through 42); this 6-week cycle is considered one course. In cycles 5 to 9, bortezomib 1.3 mg/m2 IV or subcutanously is given on days 1, 8, 22, and 29; this 6-week cycle is considered one course.[28383]
60 mg/m2 orally daily on days 1, 2, 3, and 4; bortezomib 1.3 mg/m2 subcutaneously twice weekly on weeks 1, 2, 4, and 5 of cycle 1 followed by bortezomib 1.3 mg/m2 subcutaneously once weekly on weeks 1, 2, 4, and 5 of cycles 2 to 9; and melphalan 9 mg/m2 orally daily on days 1, 2, 3, and 4 (VMP regimen) repeated every 6 weeks for 9 cycles in combination with daratumumab was evaluated in a randomized, phase 3 trial.[62907] The manufacturer recommends the following daratumumab dosage in combination with VMP: 16 mg/kg (actual body weight) IV weekly on weeks 1 to 6, 16 mg/kg IV every 3 weeks on weeks 7 to 54, and then 16 mg/kg IV every 4 weeks starting on week 55 until disease progression.[60311] In the ALCYONE trial (median follow-up of 40.1 months), the primary endpoint of PFS time was significantly higher with daratumumab plus VMP compared VMP alone (36.4 months vs. 19.3 months; hazard ratio (HR) = 0.42; 95% CI, 0.34 to 0.51; p less than 0.0001) in adult patients (n = 706; median age, 71 years; range, 40 to 93 years) with multiple myeloma who were ineligible for high-dose chemotherapy with stem-cell transplant (SCT) due to coexisting conditions or age of 65 years or older and who had not received prior systemic therapy or SCT. At the time of this analysis, the median overall survival time was significantly improved in patients in the daratumumab plus VMP arm compared with the VMP alone arm (median time not reached in either arm; HR = 0.6; 95% CI, 0.46 to 0.8; p = 0.0003).[64913]
60 mg/m2 PO daily on days 1, 2, 3, and 4 repeated every 6 weeks on cycles 1 to 9; melphalan 9 mg/m2 PO daily on days 1, 2, 3, and 4 repeated every 6 weeks on cycles 1 to 9; bortezomib 1.3 mg/m2 subcutaneously twice weekly on weeks 1, 2, 4, and 5 for the first 6-week cycle (8 doses in cycle 1) followed by bortezomib 1.3 mg/m2 subcutaneously once weekly on weeks 1, 2, 4, and 5 for 8 more 6-week cycles (4 doses/cycle in cycles 2 to 9); and 1,800 mg daratumumab and 30,000 units hyaluronidase subcutaneously weekly on weeks 1 to 6 (6 doses), every 3 weeks on weeks 7 to 54 (16 doses), and then every 4 weeks starting on week 55 until disease progression was evaluated in a single-arm cohort (n = 67) of a multicohort, open-label trial (the PLEIADES trial). The overall response rate was 88% in patients with newly diagnosed multiple myeloma who were ineligible for transplant who received daratumumab/hyaluronidase, bortezomib, melphalan, and prednisone.[65366]
Dosage not established. The progression-free survival time was not significantly improved with carfilzomib, melphalan, and prednisone compared with bortezomib, melphalan, and prednisone in a randomized, phase 3 trial (the CLARION trial); additionally, serious and fatal adverse reactions occurred more often in the carfilzomib-containing arm. There is not sufficient evidence to support the use of this drug combination for this indication.[64061]
40 to 60 mg PO once daily for 1 to 2 weeks, initially. Taper dose by 5 mg/week until 20 mg PO once daily, and then taper dose by 2.5 to 5 mg/week; the taper should generally not exceed 3 months. Guidelines state that corticosteroids are not effective for maintenance of medically-induced remission in Crohn disease and should not be used for long-term treatment. Corticosteroids for Crohn disease are more effective for small-bowel involvement than for colonic involvement. Because of the potential complications of steroid use in this disease, steroids should be used selectively and in the lowest dose possible.[64397]
40 to 60 mg PO once daily, initially. Taper dose by 5 to 10 mg/week based on clinical symptoms, cumulative steroid exposure, and onset of action of alternate therapies; limit use to the shortest duration possible with early initiation of steroid-sparing therapy. Guidelines recommend oral corticosteroids to induce remission in persons with ulcerative colitis; however, guidelines recommend against systemic corticosteroids for the maintenance of remission.[62699] [64393]
1 mg/kg PO once daily is recommended.
5 to 60 PO once daily, initially, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.14 to 2 mg/kg/day PO or 4 to 60 mg/m2/day PO in 3 to 4 divided doses. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[45339] [50175] [51324]
Current practice guidelines issued by the American Academy of Neurology and the Child Neurology Society recommend 0.75 mg/kg/day PO. If side effects (e.g., weight gain and Cushingoid facial appearance) outweigh benefits on muscle strength and function, gradual dose reduction to as low as 0.3 mg/kg/day PO can still be beneficial.[30681]
25 mg PO once daily for 10 days, or 20 mg PO once daily for 14 days, then 10 mg PO once daily for 14 days.[71474]
1 to 2 mg/kg/day (Max: 80 mg/day) PO in 1 or 2 divided doses. If 1 week or less of treatment is required, may discontinue when heart failure is controlled and inflammatory markers improve. For longer courses, taper dose by 20% to 25%/week. Usually no more than 3 weeks of corticosteroids are required.[72005] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
1 to 2 mg/kg/day (Max: 80 mg/day) PO in 1 or 2 divided doses. If 1 week or less of treatment is required, may discontinue when heart failure is controlled and inflammatory markers improve. For longer courses, taper dose by 20% to 25%/week. Usually no more than 3 weeks of corticosteroids are required.[72005] Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.5 to 1 mg/kg/dose (Max: 100 mg/dose) PO once daily for at least 4 weeks, then taper dose over 6 to 12 weeks to the lowest dose that sustains remission. Depending on disease severity, lower doses may be used.[68308] [68309] [68311] [68312] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
1 to 2 mg/kg/dose (Max: 60 mg/dose) PO once daily for at least 4 weeks, then taper dose over 12 to 24 months to the lowest dose that sustains remission.[68313] [68314] [68315] [68317] [68318] [68319] [68320] Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
12.5 to 25 mg PO once daily, initially. Taper dose to 10 mg/day within 4 to 8 weeks if tolerated, and then by 1 to 1.25 mg/day every 4 weeks once remission is achieved. For relapses, increase the dose to the prerelapse dose and taper dose more gradually.[70474] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[51324]
0.3 to 0.8 mg/kg/dose PO once daily or less, initially.[69025] Taper dose to 5 mg/day or less as quickly as possible and withdraw therapy if possible.[70847] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.5 to 2 mg/kg/dose PO once daily, initially.[65218] Taper dose to 5 mg/day or less as quickly as possible and withdraw therapy if possible.[70847] Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
40 to 80 mg PO once daily, initially. May give 60 to 100 mg PO once daily for up to first 3 days if initial intravenous glucocorticoid therapy is appropriate and not possible. Once disease is controlled, taper dose to 15 to 20 mg/day within 2 to 3 months, and then 5 mg/day or less after 1 year. In general, taper to discontinuation over 12 to 18 months. May consider a more rapid taper in those at high risk of glucocorticoid toxicity and/or receiving concomitant glucocorticoid-sparing therapy.[70292] [70293] [70294]
40 to 60 mg PO once daily, initially. When biochemical remission is achieved, taper dose by 2.5 to 5 mg/day every 2 to 4 weeks over 6 months to 5 to 10 mg/day or the lowest dose to maintain remission. Guidelines recommend prednisone monotherapy for persons with acute severe autoimmune hepatitis (AIH) followed by liver transplantation if no improvement within 2 weeks. Addition of azathioprine may be considered after cholestasis is resolved.[68993]
1 to 2 mg/kg/dose (Max: 40 to 60 mg/dose) PO once daily, initially. When biochemical remission is achieved, taper dose by 2.5 to 5 mg/day every 2 to 4 weeks over 6 months to 2.5 to 10 mg/day or the lowest dose to maintain remission.[68993] [68996] [68997] Guidelines recommend prednisone monotherapy for persons with acute severe autoimmune hepatitis (AIH) followed by liver transplantation if no improvement within 2 weeks. Addition of azathioprine may be considered after cholestasis is resolved.[68993]
20 to 40 mg PO once daily, initially. When biochemical remission is achieved, taper dose by 2.5 to 5 mg/day every 2 to 4 weeks over 6 months to 5 to 10 mg/day or the lowest dose to maintain remission. Guidelines recommend prednisone in combination with azathioprine as first-line therapy in adults who present with autoimmune hepatitis (AIH) who do not have cirrhosis, acute severe AIH, or acute liver failure. Add azathioprine after 2 weeks in persons with compensated cirrhosis. May attempt steroid withdrawal while continuing azathioprine.[68993]
1 to 2 mg/kg/dose (Max: 20 to 40 mg/dose) PO once daily, initially. When biochemical remission is achieved, taper dose by 2.5 to 5 mg/day every 2 to 4 weeks over 6 months to 2.5 to 10 mg/day or the lowest dose to maintain remission.[68993] [68996] [68997] Guidelines recommend prednisone in combination with azathioprine as first-line therapy in children who present with autoimmune hepatitis (AIH) who do not have cirrhosis, acute severe AIH, or acute liver failure. Add azathioprine after 2 weeks in persons with compensated cirrhosis. May attempt steroid withdrawal while continuing azathioprine.[68993]
0.8 mg/kg PO once daily for 7 days, in combination with melphalan; repeated every 6 weeks. The treatment combination demonstrated superior results over colchicine alone in the treatment of primary amyloidosis.[24765]
5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
2 mg/kg/dose PO once daily, initially.[55789] [55790] [55791] [55792] [55816] Higher doses of 4 to 8 mg/kg/day may be necessary.[55817] Most respond to a dose of 40 to 60 mg/day.[55818] Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
1 mg/kg/dose PO once daily (Usual dose: 60 to 100 mg/day) for 2 to 3 weeks, then reduce dose to 20 to 30 mg PO once daily over a few weeks, and then by 2.5 to 5 mg/month until discontinued within 3 to 6 months.[70696] [70697] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
40 to 80 mg/day PO in 1 to 2 divided doses for 5 to 10 days.[33558] [69016]
40 to 80 mg/day PO in 1 to 2 divided doses for 3 to 10 days.[33558] [64934] [69016]
1 to 2 mg/kg/day (Max: 40 mg/dose) PO in 1 to 2 divided doses for 3 to 10 days.[33558] [64934] [69016]
1 to 2 mg/kg/day (Max: 30 mg/dose) PO in 1 to 2 divided doses for 3 to 10 days.[33558] [64934] [69016]
1 to 2 mg/kg/day (Max: 20 mg/dose) PO in 1 to 2 divided doses for 3 to 10 days.[33558] [64934] [69016]
7.5 to 60 mg PO once daily or every other day as needed for symptom control. Use the lowest effective dose; alternate day therapy may produce less adrenal suppression.[33558] Consider add-on low dose oral corticosteroids (7.5 mg/day or less of prednisone equivalent) only for those with poor symptom control and/or frequent exacerbation despite good inhaler technique and treatment adherence. Add corticosteroids only after exclusion of other contributory factors and consideration of other add-on treatments.[69016]
7.5 to 60 mg PO once daily or every other day as needed for symptom control. Use the lowest effective dose; alternate day therapy may produce less adrenal suppression.[33558] In pediatric patients, the use of oral corticosteroids is usually limited to a few weeks until asthma control is improved and the patient can be stabilized on other, preferred treatments.[69016]
0.25 to 2 mg/kg/dose (Usual Max: 40 mg/dose) PO once daily or every other day as needed for symptom control. Use the lowest effective dose; alternate day therapy may produce less adrenal suppression. In pediatric patients, the use of oral corticosteroids is usually limited to a few weeks until asthma control is improved and the patient can be stabilized on other, preferred treatments.[33558] [69016]
0.25 to 2 mg/kg/dose (Usual Max: 30 mg/dose) PO once daily or every other day as needed for symptom control. Use the lowest effective dose; alternate day therapy may produce less adrenal suppression. In pediatric patients, the use of oral corticosteroids is usually limited to a few weeks until asthma control is improved and the patient can be stabilized on other, preferred treatments.[33558] [69016]
0.25 to 2 mg/kg/dose (Usual Max: 20 mg/dose) PO once daily or every other day as needed for symptom control. Use the lowest effective dose; alternate day therapy may produce less adrenal suppression. In pediatric patients, the use of oral corticosteroids is usually limited to a few weeks until asthma control is improved and the patient can be stabilized on other, preferred treatments.[33558] [69016]
30 to 40 mg PO once daily for 5 days.[62784] [69470] [69528] Systemic glucocorticoids shorten recovery time and improve lung function (FEV1), oxygenation, the risk of early relapse, treatment failure, and the length of hospitalization.[69470]
5 to 60 mg PO once daily, initially. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.25 mg/kg/dose to 1 mg/kg/dose PO once daily, initially.[23970] [24417] [24742] [69970] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.25 mg/kg/dose to 1 mg/kg/dose PO once daily, initially.[23970] [24417] [24742] [69970] Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
1 mg/kg/dose PO once daily, followed by a taper.[69939]
1 mg/kg/dose PO once daily, followed by a taper.[69939] [69964]
5 to 10 mg PO once daily, initially. Increase the dose by 5 mg every 7 to 10 days until symptoms improve. Max: 80 mg/day. Alternatively, 10 mg PO every other day for 3 doses, then increase the dose by 10 mg every 3 doses until symptoms improve. Max: 1.5 mg/kg/dose or 100 mg PO every other day. Taper after achieving remission for at least 2 to 3 months; reduce dose by 10 mg/month to 40 mg/dose, then by 5 mg/month to 20 mg/dose, then by 2.5 mg/month to 10 to 15 mg/dose, and then by 1 mg/month to the lowest effective dose.[61824] [71494]
0.5 mg/kg/dose PO every other day, initially. Increase the dose based on clinical response and tolerability. Max: 1.5 mg/kg/dose or 100 mg PO every other day or 1 mg/kg/dose or 60 mg PO once daily. Taper after achieving remission; reduce dose by 5 mg/month to 15 to 20 mg every other day, and then by 1 mg/month to the lowest effective dose.[71495]
5 mg PO every other day for 3 doses, then increase the dose by 5 mg every 3 doses until symptoms improve. Max: 0.75 mg/kg/dose or 50 mg PO every other day. Taper after achieving remission for at least 2 to 3 months; reduce dose by 5 mg/month to 20 mg/dose, then by 2.5 mg/month to 10 mg/dose, and then by 1 mg/month to the lowest effective dose.[61824] [66889] [71496]
0.2 to 0.5 mg/kg/dose PO once daily for 2 to 4 weeks in combination with colchicine until asymptomatic and CRP concentration is normal, then taper dose by 5 to 10 mg/day every 1 to 2 weeks for doses more than 25 mg/day, by 2.5 mg/day every 2 to 4 weeks for doses of 15 to 25 mg/day, and then by 1.25 to 2.5 mg/day every 2 to 6 weeks for doses less than 15 mg/day.[60439] [67418] [71453]
0.2 to 0.5 mg/kg/dose PO once daily for 2 to 4 weeks in combination with aspirin/NSAID and colchicine until asymptomatic and CRP concentration is normal, then taper dose by 5 to 10 mg/day every 1 to 2 weeks for doses more than 25 mg/day, by 2.5 mg/day every 2 to 4 weeks for doses of 15 to 25 mg/day, and then by 1.25 to 2.5 mg/day every 2 to 6 weeks for doses less than 15 mg/day.[60439] [67418] [71453]
Titrate to response. Usual dosage ranges from 5 to 30 mg PO once daily. Use the lowest effective dose (usually less than 7.5 mg/day, per guidelines). Usual Max: 60 mg/day PO. Guidelines for psoriasis/psoriatic arthritis recommend short-term use (avoid long-term use) of systemic corticosteroids for acute relief of symptoms/flares with caution; local corticosteroid injections are often preferable for oligoarthritis, dactylitis or in enthesitis.[50175] [51324] [62838] [63834] [63884]
There is variation in the literature with regard to dosage regimens. Prednisone 0.75 mg/kg/day to 1 mg/kg/day PO is commonly reported, followed by gradual taper over 3 to 6 weeks. Use of IV methylprednisolone for a few days may precede oral corticosteroid use. NOTE: Following biopsy to confirm diagnosis, corticosteroids are usually instituted soon afterward as an adjunctive measure; removal of the suspected offending agent /cause is the primary treatment. While many case reports suggest a possible net benefit to the use of corticosteroids, some experts advocate for more prospective study of their value.[32123]
5 to 60 mg PO once daily, initially. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
2 mg/kg/dose or 60 mg/m2/dose (Max: 80 mg/dose) PO once daily until urine is protein-free for 3 consecutive days. Then 1 to 1.5 mg/kg/dose or 40 mg/m2/dose PO every other day for 4 weeks. If needed for long-term maintenance dose, 0.5 to 1 mg/kg/dose PO every other day for 3 to 6 months.[25315] [35714] Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.3 to 1 mg/kg/dose (Max: 80 mg/dose) PO once daily for 2 weeks, then taper dose to 5 mg/day or less over a few months.[69025] [70847] [71743] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.3 to 2 mg/kg/dose (Max: 80 mg/dose) PO once daily for 4 to 6 weeks, then taper dose to 5 mg/day or less over several months.[65218] [70847] [71743] [71748] Lower doses are generally sufficient for situations of less severity, while in selected individuals higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.25 mg/kg/dose PO once every other day or less as part of a cyclosporine or tacrolimus treatment regimen.[67356]
In patients with severe skin reactions, higher initial doses (e.g., 60 mg/day PO) are usually required. Adjust until a satisfactory response is noted; taper as clinically indicated.[29779] [43319] High-dose corticosteroids are controversial; administration has been associated with decreased survival.[23971] [23972] Prednisone doses of 60 mg/day to 250 mg/day PO are equivalent to the recommended hydrocortisone doses of 240 mg/day to 1,000 mg/day.
5 to 60 mg PO once daily, initially. Adjust dose to achieve a satisfactory response, and then reduce dose by small increments to lowest dose that will maintain an adequate response. Taper long-term therapy gradually when discontinuing.[29779]
0.14 to 2 mg/kg/dose PO once daily, initially. Adjust dose to achieve a satisfactory response, and then reduce dose by small increments to lowest dose that will maintain an adequate response. Taper long-term therapy gradually when discontinuing.[29779] [55575]
40 to 100 mg PO once daily for 1 to 3 weeks until symptomatic control. May follow with 40 to 100 mg PO every other day, with tapering by 5 to 10 mg/month.[55471] [55473] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated.[50175] [51324] Corticosteroids are not indicated as initial treatment for anaphylaxis, but can be given as adjunctive therapy after the administration of epinephrine.[66106] [64564] [70421] [70422]
1 to 2 mg/kg/day PO in 1 to 4 divided doses (Max: 60 mg/day) for 1 to 3 weeks until symptomatic control. Treatment duration is dependent on specific allergic/hypersensitivity condition, usually 2 to 3 weeks.[55475] [55476] [55477] [55478] May follow with 1 to 2 mg/kg/day PO every other day, with tapering by 5 to 10 mg/month.[55471] [55472] [55473] Corticosteroids are not indicated as initial treatment for anaphylaxis, but can be given as adjunctive therapy after the administration of epinephrine.[66106] [64564] [64934] [70421] [70422]
Short courses of 30 to 50 mg/day PO can be given during the late phase of an acute reaction, once oral therapy is appropriate.[24005] FDA-approved dosage: 5 to 60 mg/day PO, depending on disease severity for angioedema; taper as clinically indicated.[29779] [43319]
Corticosteroid use in ARDS is controversial. If there are no signs of improvement 7 to 14 days after ARDS onset, 2 mg/kg/day to 4 mg/kg/day PO for 7 to 14 days has been recommended.[23999]
The initial dosage may vary from 5 to 60 mg PO per day. Guidelines use a dose of 0.5 mg/kg/day PO for 4 weeks, then 0.25 mg/kg/day PO for 8 weeks. Taper to 0.125 mg/kg/day or 0.25 mg/kg/day PO on alternate days. Guidelines suggest use of prednisone with cyclophosphamide or azathioprine, and a minimum of 6 months duration. Objective responses may not be noted until at least 3 months of therapy. Exact duration of treatment and need for long-term maintenance should be individualized to clinical response and tolerance of therapy. Chronic doses of prednisone (15 mg to 20 mg PO once daily) may be adequate as maintenance therapy.[26496] [51324]
The initial dosage may vary from 5 to 60 mg PO per day.[51324] Gradually taper after 1 to 2 weeks and discontinue by 4 to 6 weeks, guided by symptoms.
The initial dosage may vary from 5 to 60 mg PO per day.[51324] Weight-based dosing: 0.14 mg/kg to 2 mg/kg (4 to 60 mg/m2) PO daily, given in 1 to 4 divided doses. Gradually taper after 1 to 2 weeks and discontinue by 4 to 6 weeks, as guided by symptoms.
40 mg/m2/day PO on Day 1 through Day 22, then taper. Chemotherapy cycle is repeated every 57 days.
40 mg/m2/day PO on Day 1 through Day 14; cycle is repeated every 28 days.
20 mg/m2 orally twice daily on days 1 to 7 in combination with brentuximab vedotin 1.8 mg/kg (not to exceed 180 mg/dose) IV on day 1; doxorubicin 25 mg/m2 IV on days 1 and 2; vincristine 1.4 mg/m2 IV on day 8; etoposide 125 mg/m2 IV on days 1, 2, and 3; and cyclophosphamide 600 mg/m2 IV on days 1 and 2 repeated every 3 weeks for up to 5 cycles. Administer primary prophylaxis with a granulocyte colony-stimulating factor starting in cycle 1 due to the high incidence of febrile neutropenia.[45378] At a median follow-up time of 42.1 (range, 0.1 to 80.9) months, the 3-year event-free survival rate was significantly improved in patients (median age, 15.6 years; range, 3.4 to 21.99 years) with newly diagnosed, stage IIB with bulk tumor or stage IIIB, IVA, or IVB classic Hodgkin lymphoma who received brentuximab vedotin plus AVEPC compared with doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) (92.1% vs. 82.5%; hazard ratio = 0.41; 95% CI, 0.25 to 0.67) in a multicenter, randomized, phase 3 trial (n = 587). The 3-year overall survival rates were 99.3% and 98.5% in the brentuximab vedotin plus AVEPC and ABVE-PC arms, respectively.[68172]
A range of 40 mg/day to 80 mg/day PO is suggested. Higher quality data are needed to establish the benefits vs. risks and optimal dose and duration of therapy. Experts generally agree that patients who have neurologic deficits should receive a corticosteroid; many patients with MSCC require corticosteroids to help preserve neurologic function, such as ambulation.[24582] [51639]
5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals, higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.14 to 2 mg/kg/day PO or 4 to 60 mg/m2/day PO in 3 to 4 divided doses. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[45339] [50175] [51324]
1 to 1.5 mg/kg/dose (Max: 80 mg/dose) PO once daily, initially. Taper dose by 1 to 10 mg/day every 7 to 28 days as inflammation resolves.[71021] [71025] [71027] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected individuals, higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
1 to 2 mg/kg/dose (Max: 80 mg/dose) PO once daily, initially. Taper dose to 0.15 mg/kg/day (Max: 5 mg/day) or less within 4 weeks; limit use to 3 months or less.[71015] [71016] [71021] [71024] [71025] [71027] Lower doses are generally sufficient for situations of less severity, while in selected individuals, higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
5 mg to 60 mg PO per day, administered in 1 to 4 divided doses, depending upon disease being treated. Depending on the indication, the initial dose may be gradually tapered after 1 to 2 weeks and discontinued by 4 to 6 weeks, as guided by symptoms.
0.14 to 2 mg/kg/day PO or 4 to 60 mg/m2/day PO, given in 4 divided doses. Depending on indication, gradually taper the initial dose after 1 to 2 weeks and discontinue by 4 to 6 weeks, guided by symptoms.
60 mg PO once daily for 5 days, then reduce dose by 10 mg/day every day for 5 days for a total treatment duration of 10 days.[56392] Alternatively, 25 mg PO twice daily for 10 days.[41527] Steroids are effective in increasing the probability of complete facial functional recovery.[55538]
The optimal dose of prednisone for infantile spasms has not been determined. The most frequently reported doses in the literature range from 1 mg/kg/day to 3 mg/kg/day PO. One study comparing low dose IM ACTH (20 International Units/m2) with prednisone 2 mg/kg/day PO reported no significant difference in response rates between the groups (spasm cessation in 42% and 33% of patients respectively).[32957] Other studies using higher doses of IM ACTH (150 International Units/m2) in patients ranging from 2 to 21 months of age have shown ACTH therapy to be superior to prednisone.[32958] [32959] Based on the evidence currently available, the American Academy of Neurology and the Child Neurology Society's practice parameters for the treatment of infantile spasms state that there is insufficient evidence that oral corticosteroids are effective in the treatment of infantile spasms.[32960]
There are limited data available for the treatment of refractory seizure types in pediatric patients. The optimal dose of prednisone for adjunctive therapy of seizure disorders has not been determined. Doses of 0.3 mg/kg/day to 3 mg/kg/day PO have been used. One case series of 28 pediatric patients ages 2 to 10 years suggests that prednisone therapy may be an effective adjunct treatment for intractable generalized epilepsy.[32899] Prednisone 1 mg/kg/day PO was administered for 12 weeks in addition to each patient's regular anticonvulsant regimen. Per parent diary, almost half of the study patients became seizure free, 36% had more than a 50% decrease in seizure frequency, and 18% had no change in seizure frequency. Treatment was most beneficial in those with absence seizures and early Lennox-Gastaut syndrome. In another retrospective case series, 32 mentally retarded children received various steroids for intractable epilepsy. Eight of those, ages 9 months to 6 years, received prednisone at varying doses and duration (0.3 to 3 mg/kg/day for a duration of 7 days to 24 months).[32961] Two patients had 100% reduction in seizure frequency, 1 patient had a 50% to 75% reduction, and 5 patients had no change in seizure frequency as reported by parents and confirmed with EEG. All 3 patients who responded had complex partial seizures. Of those 3 patients, 2 relapsed in less than 1 month after prednisone discontinuation. A non-randomized, non-blinded study compared IM ACTH 150 International Units/m2 for 1 week followed by an 11-week taper to prednisone 3 mg/kg/day for 4 weeks followed by 3 mg/kg every other day for 8 weeks, and then a 4-week taper. Infants and children with infantile spasms and children with other types of non-specified intractable seizures were included in the analysis.[32958] The mean age of patients in the non-specified intractable seizures group was 42.5 months. The investigators found that prednisone was effective in 59% (n = 13) of patients with infantile spasms who had a hypsarrhythmic EEG abnormality. Prednisone was reported to be ineffective in all 30 patients with other seizure types.
Guidelines recommend 1 mg/kg/day to 3 mg/kg/day PO for 3 to 5 days for asymptomatic mild or moderate acute cellular rejection (ISHLT 1R or 2R). A corticosteroid taper may be considered. Not first-line for symptomatic rejection (ISHLT 1R, 2R, or 3R) or for asymptomatic severe rejection (ISHLT 3R).[51803]
Titrate to response. Various dosage regimens are reported in the literature.[51803] [69984] [69985] [69986] [69987] One institution reported a dose of 1 mg/kg/day in 2 divided doses, tapering to 0.05 mg/kg/day by 6 to 12 months. The same institution reports tapering prednisone dose to 10 mg PO daily by 3 months post-transplant, 5 mg PO daily by 6 months post-transplant, and then reduce by 1 mg per month to discontinuation.[69987] An alternative regimen reported in the literature is 0.5 mg to 1 mg/kg/day in the first week post-transplant, then tapering to 0.15 mg/kg/day by 3 months, and further tapering to 0.1 mg/kg/day with goal to completely discontinue therapy by 6 months.[69986] Consider endomyocardial biopsy during dose reduction to assess asymptomatic rejection, especially in individuals at higher risk. Guidelines state corticosteroid avoidance, early corticosteroid weaning, or very low dose maintenance corticosteroid therapy are all acceptable approaches.[51803] [69984]
20 to 40 mg PO once daily for 4 to 8 weeks can be considered for patients with moderate to severe immune reconstitution inflammatory syndrome (IRIS).[34362]
20 mg PO twice daily starting after 48 to 72 hours of anti-Toxoplasma therapy, followed by a rapid taper for severe chorioretinitis in vision-threatening area.[67495] [70821]
0.5 mg/kg/dose (Max: 20 mg/dose) PO twice daily starting after 48 to 72 hours of anti-Toxoplasma therapy, followed by a rapid taper for severe chorioretinitis in vision-threatening area.[61724] [70821]
0.5 mg/kg/dose PO twice daily starting after 48 to 72 hours of anti-Toxoplasma therapy for individuals with cerebrospinal fluid (CSF) protein of 1 g/dL or more or severe chorioretinitis in vision-threatening area. Continue until CSF protein is less than 1 g/dL or resolution of severe chorioretinitis.[61724] [70821]
0.5 mg/kg/dose PO twice daily starting after 48 to 72 hours of anti-Toxoplasma therapy for individuals with cerebrospinal fluid (CSF) protein of 1 g/dL or more or severe chorioretinitis in vision-threatening area. Continue until CSF protein is less than 1 g/dL or resolution of severe chorioretinitis.[61724] [70821]
60 mg/m2 orally on days 1, 2, 3, 4, and 5 in combination with gemcitabine (800 mg/m2 IV over 30 minutes on days 1 and 8), cisplatin (25 mg/m2 IV on days 1, 2, and 3), and thalidomide (200 mg orally daily) repeated every 21 days until disease progression or for up to 6 cycles was evaluated in patients with previously untreated PTCL in a randomized trial. Patients received aspirin 100 mg orally daily during thalidomide therapy. The use of granulocyte colony-stimulation factor was permitted as indicated.[62321]
100 mg orally daily on days 1, 2, 3, 4, and 5 in combination with brentuximab vedotin 1.8 mg/kg (not to exceed 180 mg/dose) IV on day 1, cyclophosphamide 750 mg/m2 IV on day 1, and doxorubicin 50 mg/m2 IV on day 1 given every 21 days for 6 to 8 cycles of therapy. The progression-free survival time (evaluated via an independent review facility) was significantly improved in patients with CD30-expressing systemic anaplastic large-cell lymphoma (sALCL) or PTCL who received brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (CHP) compared with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (48.2 months vs. 20.8 months; hazard ratio (HR) = 0.71; 95% CI, 0.54 to 0.93) in a multicenter, randomized, double-blind, phase 3 trial (the ECHELON-2 trial; n = 452). Overall survival was also significantly improved in the brentuximab vedotin-containing arm (HR = 0.66; 95% CI, 0.46 to 0.95). In this trial, 70% of patients had sALCL and 30% of patients had PTCL (e.g., including PTCL not otherwise specified (16%), angioimmunoblastic T-cell lymphoma (12%), adult T-cell leukemia/lymphoma (2%), and enteropathy-associated T-cell lymphoma (less than 1%)).[45378]
2 mg/kg/day PO in 3 divided doses until CRP is normalized, then taper over 2 to 3 weeks. This regimen, administered after an initial course of IV steroids that is continued until the patient is afebrile and concurrently with IVIG (2 grams/kg IV once) and aspirin, may be considered for primary treatment of high-risk patients with acute disease or in the retreatment of patients who have recurrent or recrudescent fever after initial IVIG treatment.[61950] [61963]
100 mg orally daily on days 1, 2, 3, 4, and 5 in combination with brentuximab vedotin 1.8 mg/kg (not to exceed 180 mg/dose) IV on day 1, cyclophosphamide 750 mg/m2 IV on day 1, and doxorubicin 50 mg/m2 IV on day 1 given every 21 days for 6 to 8 cycles of therapy. The progression-free survival (PFS) time (evaluated via an independent review facility) was significantly improved in patients with CD30-expressing sALCL or peripheral T-cell lymphoma who received brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (CHP) compared with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (48.2 months vs. 20.8 months; hazard ratio (HR) = 0.71; 95% CI, 0.54 to 0.93) in a multicenter, randomized, double-blind, phase 3 trial (the ECHELON-2 trial; n = 452). Overall survival was also significantly improved in the brentuximab vedotin-containing arm (HR = 0.66; 95% CI, 0.46 to 0.95). In patients with sALCL (n = 314; anaplastic lymphoma kinase (ALK)-negative sALCL, 48%; ALK-positive sALCL, 22%), the PFS times were 55.7 months and 54.2 months in patients who received brentuximab vedotin plus CHP and CHOP, respectively (HR = 0.59; 95% CI, 0.42 to 0.84).[45378]
40 to 60 mg PO 2 to 3 times daily; taper dose after 5 to 7 days over 1 to 2 weeks. A suggested taper is 40 mg PO twice daily on days 1 to 5; then 40 mg PO once daily on days 6 to 10; then 20 mg PO once daily on days 11 to 21. Start therapy as early as possible and within 72 hours after starting specific PCP therapy.[34362] [64856] [64858] [64860] [64862] [64907]
40 to 60 mg PO 2 to 3 times daily; taper dose after 5 to 7 days over 1 to 2 weeks. A suggested taper is 40 mg PO twice daily on days 1 to 5; then 40 mg PO once daily on days 6 to 10; then 20 mg PO once daily on days 11 to 21. Start therapy as early as possible and within 72 hours after starting specific PCP therapy.[34362] [64856] [64858] [64860] [64862] [64907]
1 mg/kg/dose PO twice daily on days 1 to 5; then 0.5 to 1 mg/kg/dose PO twice daily on days 6 to 10; then 0.5 mg/kg/dose PO once daily on days 11 to 21. Start therapy as early as possible and within 72 hours after starting specific PCP therapy.[34361] [64856] [64858] [64860] [64862] [64907]
2.67 mg/kg/dose PO once daily or 60 to 120 mg PO once daily with a taper over 6 to 8 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[43319] [61094] [69585] [69587] [69589]
2 to 4 mg/kg/dose (Max: 60 mg/dose) PO once daily for 4 to 6 weeks, then taper over 2 to 4 weeks. Guidelines recommend as adjunct therapy for meningitis. Routine use outside of CNS involvement is not recommended; however, select patients may benefit.[34361] [43319] [61094] [69585] [69586] [69587] [69589] [70821]
2 to 3 mg/kg/day PO for 4 to 12 weeks, then gradually taper dose to complete therapy by 9 to 12 months of age. Higher doses of 5 mg/kg/day and shorter durations (1 to 6 weeks) with intermittent courses as needed have been used. Oral corticosteroids are recommended in individuals that do not respond to or have a contraindication to oral propranolol.[63864] [63885] [72240]
2 to 3 mg/kg/day PO for 4 to 12 weeks, then gradually taper dose to complete therapy by 9 to 12 months of age. Higher doses of 5 mg/kg/day and shorter durations (1 to 6 weeks) with intermittent courses as needed have been used. Oral corticosteroids are recommended in individuals that do not respond to or have a contraindication to oral propranolol.[63864] [63885] [72240]
100 mg orally daily on days 1, 2, 3, 4, and 5 in combination with polatuzumab vedotin 1.8 mg/kg IV, rituximab 375 mg/m2 IV, cyclophosphamide 750 mg/m2 IV, and doxorubicin 50 mg/m2 IV on day 1 repeated every 21 days for 6 cycles has been evaluated in a randomized, double-blind, placebo-controlled, phase 3 trial (n = 879; the POLARIX trial). Rituximab 375 mg/m2 IV was continued for 2 additional cycles of therapy (cycles 7 and 8).[67350]
60 mg PO once daily for 1 to 2 days.[67513] [67515]
2 mg/kg/dose (Max: 60 mg/dose) PO once daily for 1 to 2 days.[63307] [67513]
40 mg PO once daily for 7 days.[57437]
40 to 60 mg PO once daily, initially. Taper dose based on clinical course.[67717] [67718] [67719] [67720] [67721]
40 to 60 mg PO once daily, initially. Taper dose based on clinical course.[67717] [67718] [67719] [67720] [67721] [67728] [67729]
1 mg/kg/dose (Max: 60 to 80 mg/dose) PO once daily for 3 to 5 days.[67800] [67806] [67913] [67914] [67915]
1 mg/kg/dose (Max: 60 to 80 mg/dose) PO once daily for 3 to 5 days.[67801] [67806] [67913] [67914] [67915]
0.5 to 2 mg/kg/dose (Max: 60 mg/dose) PO once daily, followed by an extended taper over up to 12 months.[67800] [67917]
0.5 to 2 mg/kg/dose (Max: 60 mg/dose) PO once daily, followed by an extended taper over up to 12 months.[67800] [67801] [67917]
40 mg PO once daily for 1 to 2 weeks, followed by a gradual taper over 2 to 4 weeks or more depending on clinical response.[61515] The FDA-approved dosage is 5 to 60 mg/day.[29779]
40 mg PO once daily for 2 to 4 weeks, followed by a gradual taper over 2 to 3 months depending on clinical response.[61515] The FDA-approved dosage is 5 to 60 mg/day.[29779]
5 to 10 mg PO once daily, initially. Taper dose to the lowest effective dose. Doses more than 10 mg/day are rarely indicated.[29779] [51324] [68410]
10 mg PO 3 times daily for up to 12 weeks, followed by a taper.[68505] [68548]
60 to 80 mg/day PO administered in two divided doses and tapered over 1 to 2 weeks has been used to treat complications including airway obstruction due to tonsillar enlargement; autoimmune hemolytic anemia, severe thrombocytopenia, and aplastic anemia; CNS involvement; myocarditis; and pericarditis.[68592]
40 mg PO once daily for 4 weeks. Assess response using Lille score on Day 4 or 7 and discontinue therapy in nonresponders (Lille score more than 0.45). Severe alcohol-associated hepatitis is defined as Maddrey discriminant function [MDF] of 32 or more or model for end-stage liver disease [MELD] score more than 20.[68874] [71716]
60 mg PO once daily for days 1 to 7; 30 mg PO once daily for days 8 to 14; 15 mg PO once daily for days 15 to 21.[38870]
1 to 2 mg/kg/day PO starting 3 days before antiparasitics and continuing for the duration of therapy. Titrate based on clinical response. Taper over 6 to 8 weeks after antiparasitic therapy is complete to avoid rebound symptoms.[63735] [69053] [69054] [69056] [69057]
1 to 2 mg/kg/day PO starting 3 days before antiparasitics and continuing for the duration of therapy. Titrate based on clinical response. Taper over 6 to 8 weeks after antiparasitic therapy is complete to avoid rebound symptoms .[63735] [69053] [69054] [69056] [69057]
0.5 to 1 mg/kg/dose PO once daily.[69154] [69164] [71082] Alternatively, 20 mg PO once daily and reduce dose to 10 to 15 mg/day if needed based on tolerability.[71083]
0.5 to 1 mg/kg/dose PO once daily.[71082]
30 to 40 mg PO once daily, initially.[64373] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
60 mg PO once daily, initially, followed by a gradual taper over weeks to months. Corticosteroid therapy duration has been reported from 28 days to 6 months or longer.[69341] [69352]
0.5 mg/kg/dose PO once daily for 1 to 2 weeks, then 0.5 mg/kg/dose PO every other day for 6 to 8 weeks before tapering dose by 5 to 10 mg every 2 weeks to discontinue. Alternatively, 0.75 mg/kg/dose PO once daily for 6 weeks, then 0.5 mg/kg/dose PO once daily for 6 weeks before tapering dose by 5 mg every 6 weeks for a total duration of at least 6 to 12 months. Wean steroids based on clinical response and serum IgE concentrations.[29779] [61353] [68937]
0.5 mg/kg/dose PO once daily for 1 to 2 weeks, then 0.5 mg/kg/dose PO every other day for 6 to 8 weeks before tapering dose by 5 to 10 mg every 2 weeks to discontinue. Alternatively, 0.75 mg/kg/dose PO once daily for 6 weeks, then 0.5 mg/kg/dose PO once daily for 6 weeks before tapering dose by 5 mg every 6 weeks for a total duration of at least 6 to 12 months. Wean steroids based on clinical response and serum IgE concentrations.[29779] [61353] [68937]
1 to 2 mg/kg/dose PO once daily for 1 to 2 weeks, then taper dose over 2 weeks. A longer course (up to 6 months) may be necessary in severe disease.[67356] [69669] [69670]
1 to 2 mg/kg/dose PO once daily for 1 to 2 weeks, then taper dose over 2 weeks. A longer course (up to 6 months) may be necessary in IgA vasculitis-associated nephritis with nephrotic syndrome and/or rapidly deteriorating kidney function.[37071] [55474] [67356] [69669]
1,250 mg PO once daily for 3 to 5 days.[69791] [69809]
15 to 40 mg PO once daily, initially. Taper dose to lowest effective dose, typically 10 mg/day or less. There is no clear benefit of extending treatment beyond 2 years.[69853] [69854] The FDA-approved dosage is 5 to 60 mg/day.[29779]
Titrate to response. Usual dose is 10 mg to 20 mg PO daily; 0.3 mg/kg/day has also been reported (dose varies according to institution, etiology of liver disease, and history of rejection). Guidelines recommend slowly tapering the dose with the goal of drug discontinuation; the majority of patients should be discontinued from prednisone 3 months post-transplantation. Long-term, low-dose prednisone therapy should be considered for patients with higher immunological risk (e.g., history of steroid-resistant rejection, immune-mediated diseases).[69988] [69989] [69990] [69991] [69992]
1 mg/kg/dose PO once or twice daily. Systemic corticosteroids are not generally recommended for the treatment of eosinophilic esophagitis; swallowed topical corticosteroids are preferred. The efficacy of systemic corticosteroids is similar to swallowed topical corticosteroids; however, the risk for adverse events is higher. Systemic corticosteroids may be useful if swallowed topical corticosteroids are not effective or rapid improvement in symptoms is required. Reserve use of systemic steroids for emergency situations with severe dysphagia (stricturing disease) or significant weight loss.[55346] [56033] [65816] [70029] [70042] [70043]
1 mg/kg/dose PO once or twice daily. Systemic corticosteroids are not generally recommended for the treatment of eosinophilic esophagitis; swallowed topical corticosteroids are preferred. The efficacy of systemic corticosteroids is similar to swallowed topical corticosteroids; however, the risk for adverse events is higher. Systemic corticosteroids may be useful if swallowed topical corticosteroids are not effective or rapid improvement in symptoms is required. Reserve use of systemic steroids for emergency situations with severe dysphagia (stricturing disease) or significant weight loss.[55346] [56033] [65816] [70029] [70042] [70043]
1 mg/kg/day (Usual max: 60 mg/day) PO once daily, followed by an extended taper. Dose and duration determined based on clinical response.[70278] [70280] [71169] [71170]
1 mg/kg/day PO for 1 to 3 weeks, followed by a taper over at least 1 month. Average taper has been reported as 5 months.[70278] [70280] [71170]
0.35 mg/kg/dose (Max: 60 mg/dose) PO once daily for up to 18 weeks, followed by a taper.[71195] [71196]
40 to 80 mg PO once daily for 5 to 7 days, then tapered to discontinuation over 2 to 6 months.[53390]
1 mg/kg/day (Max: 80 mg/day) PO for 5 to 7 days, then tapered to discontinuation over 2 to 6 months.[53390] [70821]
40 to 80 mg PO once daily tapered to the lowest dose required to control the reaction; initiate a slow taper to discontinuation after the reaction is controlled.[53390]
1 mg/kg/day (Max: 80 mg/day) PO tapered to the lowest dose required to control the reaction; initiate a slow taper to discontinuation after the reaction is controlled.[53390] [70821]
1 mg/kg/dose (Usual dose: 40 to 60 mg/dose) PO once daily until resolution of nodules.[71381] [71383] [71384] [71385]
0.5 mg/kg/dose PO once daily, initially, starting after IV methylprednisolone. Taper dose by one-half every 5 days based on clinical response.[71449]
2 mg/kg/dose PO once daily for 4 to 14 days starting after IV methylprednisolone, followed by taper over at least 2 weeks.[71451]
0.5 to 1 mg/kg/dose (Max: 80 mg/dose) PO once daily, initially. Taper dose to 5 mg/day by 4 to 6 months. Guide duration of therapy based on clinical response and tolerability.[67356] [71444] [71445]
1 to 2 mg/kg/dose (Max: 60 mg/dose) PO once daily for 2 to 4 weeks, then taper dose to 10 to 15 mg/day by 12 weeks and 0 to 10 mg/day by 6 months. Guide duration of therapy based on clinical response and tolerability.[71444] [71446]
1 to 2 mg/kg/dose (Max: 60 mg/dose) PO once daily for 2 to 4 weeks, then taper dose to less than 0.5 mg/kg/day by 12 weeks and less than 0.2 mg/kg/day by 6 months. Guide duration of therapy based on clinical response and tolerability.[71444] [71446]
5 to 7.5 mg PO once daily for 2 years, then reduce dose by 1 mg/day every 2 months.[67356]
25 mg PO once daily for 15 days, then 12.5 mg PO once daily for 15 days, then 6.25 mg PO once daily for 15 days, and then 6.25 mg PO every other day for 15 days.[71608] [71627]
0.5 to 1.5 mg/kg/dose PO once daily, initially, until disease control is achieved or up to the end of the consolidation phase. Taper dose by 10% to 25% every 2 to 3 weeks until 15 to 25 mg/day, then by 1 to 5 mg/dose every 3 to 4 weeks, or alternately by 2.5 mg/dose every week until 10 mg/day and then by 1 mg/dose every week.[71629] The FDA-approved initial dosage is 5 to 60 mg/day PO, depending on the disease being treated. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[50175] [51324]
0.14 to 2 mg/kg/day PO or 4 to 60 mg/m2/day PO in 3 to 4 divided doses. Lower doses are generally sufficient for situations of less severity, while in selected persons higher initial doses may be required. Continue or adjust the initial dosage until a satisfactory response is noted. After a favorable response is noted, determine the maintenance dose by decreasing the dose in small decrements at appropriate intervals until the lowest dose which will maintain an adequate clinical response is reached. If discontinuing after long-term therapy, withdraw the drug gradually rather than abruptly.[45339] [50175] [51324] [55575] [61937]
0.5 to 1 mg/kg/dose PO once daily, initially, until disease control is achieved or up to the end of the consolidation phase. Taper dose by 10% to 25% every 2 to 3 weeks until 15 to 25 mg/day, then by 1 to 5 mg/dose every 3 to 4 weeks, or alternately by 2.5 mg/dose every week until 10 mg/day and then by 1 mg/dose every week.[71628] [71629]
0.5 to 1 mg/kg/dose PO once daily, initially, until disease control is achieved or up to the end of the consolidation phase. Taper dose by 10% to 25% every 2 to 3 weeks until 15 to 25 mg/day, then by 1 to 5 mg/dose every 3 to 4 weeks, or alternately by 2.5 mg/dose every week until 10 mg/day and then by 1 mg/dose every week.[71628] [71629]
1 to 1.5 mg/kg/dose PO once daily, initially, until disease control is achieved or up to the end of the consolidation phase. Taper dose by 10% to 25% every 2 to 3 weeks until 15 to 25 mg/day, then by 1 to 5 mg/dose every 3 to 4 weeks, or alternately by 2.5 mg/dose every week until 10 mg/day and then by 1 mg/dose every week.[71628] [71629]
25 mg PO once daily for 3 to 6 days followed by a taper over 2 to 3 weeks.[71722] [71723]
1.5 to 2 mg/kg/dose (Max: 25 mg/dose) PO once daily for 3 to 6 days followed by a taper over 2 to 3 weeks.[71722] [71723] [71724]
1.5 to 2 mg/kg/dose PO once daily for 3 weeks followed by a taper.[71723] [71724]
1.5 to 2 mg/kg/dose PO once daily for 3 weeks followed by a taper.[71723] [71724]
0.5 mg/kg/dose (Max: 40 mg/day) PO once daily; continue until resolution of symptoms (Max: 3 weeks). After resolution of symptoms maintain dose for 3 to 5 days then taper by 5 mg/day every 7 days.[71725] [71726] [71730] [71731]
0.5 mg/kg/dose (Max: 40 mg/day) PO once daily; continue until resolution of symptoms (Max: 3 weeks). After resolution of symptoms maintain dose for 3 to 5 days then taper by 5 mg/day every 7 days.[71725] [71726] [71730] [71731]
0.5 mg/kg/day PO, initially. Taper dose over 6 to 12 weeks. Some individuals may be unable to maintain treatment response as the dose is reduced.[71863]
0.5 mg/kg/day PO, initially. Taper dose over 6 to 12 weeks. Some individuals may be unable to maintain treatment response as the dose is reduced.[71863]
2.5 to 10 mg/kg/day PO for 3 days for 3 months, 200 mg PO once weekly for 3 months, 60 mg PO twice weekly for 3 months, or 300 mg PO once monthly for 4 months. Retreatment with 1,000 mg PO as a single dose has been used for resistant widespread alopecia areata.[71991] [71992]
2.5 to 10 mg/kg/day PO for 3 days for 3 months, 60 mg PO twice weekly for 3 months, or 300 mg PO once monthly for at least 3 months.[71992] [71993] [71994]
2.5 to 10 mg/kg/day PO for 3 days for 3 months, or 30 mg PO twice weekly for 3 months, or 300 mg PO once monthly for at least 3 months.[71871] [71992] [71993] [71994]
1 to 2 mg/kg/day (Max: 80 mg/day) PO in 1 or 2 divided doses for 2 to 4 weeks followed by a taper.[72005] [72024]
1 mg/kg/dose (Max: 50 mg/dose) PO once daily for 9 days, then decrease the dose by 25% daily each day for the next 3 days.[62372] [72236]
1 mg/kg/dose (Max: 50 mg/dose) PO once daily for 9 days, then decrease the dose by 25% daily each day for the next 3 days.[62372] [72236]
Prednisone is a prodrug and bioactivation to prednisolone occurs in the liver, but even in severe hepatic disease this bioactivation appears to be nearly complete. No specific dosage adjustment appears to be necessary in patients with hepatic disease. The use of prednisolone instead of prednisone has been preferred historically for patients with severe hepatic impairment, but most pharmacokinetic data suggest there is no basis for this preference.[55840][55461][68680] Doses are equivalent (i.e., 1 mg prednisone is equivalent to 1 mg of prednisolone).[54137]
Specific guidelines for dosage adjustments in renal impairment are not available; it appears that no dosage adjustments are needed.
† Off-label indication




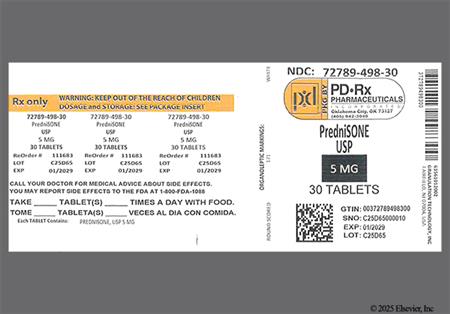



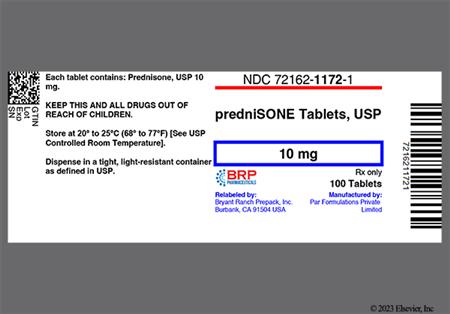












Prednisone is the most commonly-prescribed oral corticosteroid. The drug is metabolized in the liver to its active form, prednisolone. Relative to hydrocortisone, prednisone is roughly 4 times as potent as a glucocorticoid. Prednisone is used in many conditions in adult and pediatric patients, including allograft rejection, asthma, chronic obstructive pulmonary disease (COPD), systemic lupus erythematosus (SLE), rheumatoid and psoriatic arthritis, and many other allergic, dermatologic, and inflammatory states. Prednisone has very little mineralocorticoid activity, so it is not used in the management of adrenal insufficiency unless a more potent mineralocorticoid is administered concomitantly. Systemic corticosteroids may be added to other long-term maintenance medications in the management of uncontrolled severe persistent asthma. Once stabilization of asthma is achieved, regular attempts should be made to reduce or eliminate the use of systemic corticosteroids due to the side effects associated with chronic administration. Short courses of treatment may be used in moderate to severe exacerbations of asthma.[69016][66299] Short courses of systemic corticosteroids such as prednisone have particular benefits in treating acute exacerbations of COPD.[62784][69470] If long-term therapy with prednisone is required for any indication, the lowest possible effective dose should be used.
Updates for coronavirus disease 2019 (COVID-19):
The World Health Organization strongly recommends the use of systemic corticosteroids, including prednisone, in patients with severe or critical COVID-19; but suggests against use in patients with non-severe COVID-19.[65876] The National Institutes of Health (NIH) COVID-19 treatment guidelines recommend using another corticosteroid, dexamethasone, in hospitalized patients with COVID-19 who require supplemental oxygen, including those on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO); however, prednisone may be used as an alternative corticosteroid if dexamethasone is unavailable. The NIH recommends against the use of corticosteroids in patients with mild to moderate COVID-19 (i.e., non-hospitalized patients or hospitalized patients that do not require supplemental oxygen).[65314]
For storage information, see the specific product information within the How Supplied section.
Glucocorticoids, such as prednisone, are responsible for protein metabolism, and prolonged therapy can result in various musculoskeletal manifestations, including: myopathy (myalgia, muscle wasting, muscle weakness, and quadriparesis), arthralgia, impaired wound healing, tendon rupture (particularly affecting the Achilles tendon), bone matrix atrophy (osteoporosis and osteopenia), bone fractures such as vertebral compression fractures or fractures of long bones, and avascular necrosis of femoral or humoral heads. These effects are more likely to occur in older or debilitated patients. Of note, abrupt cessation of corticosteroids can cause arthralgia and myalgia. Glucocorticoids interact with calcium metabolism at many sites, including: decreasing the synthesis by osteoblasts of the principle proteins of bone matrix, malabsorption of calcium in both the nephron and the gut, and reduction of sex hormone concentrations. Although all of these actions probably contribute to glucocorticoid-induced osteoporosis, the actions on osteoblasts is most important. Glucocorticoids do not modify vitamin D metabolism.[24837] Postmenopausal women, in particular, should be monitored for signs of osteoporosis during corticosteroid therapy. Because of retardation of bone growth, children receiving prolonged corticosteroid therapy may have growth inhibition.[43319] [51324]
Corticosteroid therapy, including prednisone therapy, can mask the symptoms of infection and should be avoided during an acute viral, fungal, or bacterial infection. Leukocytosis is a common physiologic effect of systemic corticosteroid therapy and may need to be differentiated from the leukocytosis that occurs with inflammatory or infectious processes.[30943] [65096] [65097] Neutropenia, including febrile neutropenia, has been reported by recipients of corticosteroids. Immunosuppression is most likely to occur in patients receiving high-dose (e.g., equivalent to 1 mg/kg or more of prednisone daily), systemic corticosteroid therapy for any period of time, particularly in conjunction with corticosteroid-sparing drugs (e.g., troleandomycin) and/or concomitant immunosuppressant agents; however, patients receiving moderate dosages of systemic corticosteroids for short periods or low dosages for prolonged periods also may be at risk. Corticosteroids can reactivate tuberculosis and should not be used in patients with a history of active tuberculosis except when chemoprophylaxis is instituted concomitantly. Patients receiving immunosuppressive doses of corticosteroids should be advised to avoid exposure to measles or varicella (chickenpox) and, if exposed to these diseases, to seek medical advice immediately. Additionally, health care providers should monitor prednisone recipients for signs of an opportunistic fungal infection as cases of oropharyngeal candidiasis have been reported with the use of corticosteroids. The development of Kaposi's sarcoma has been associated with prolonged administration of corticosteroids, such as prednisone. Discontinuation of prednisone may result in clinical improvement.[43319] [51324]
Corticosteroids are divided into two classes: mineralocorticoids and glucocorticoids. Prednisone is a glucocorticoid with minimal mineralocorticoid activity. Mineralocorticoids alter electrolyte and fluid balance by facilitating sodium retention and hydrogen and potassium excretion at the level of the distal renal tubule, resulting in increased plasma volume. Although the incidence may vary by study design and population, mineralocorticoid properties of prednisone can cause fluid retention; electrolyte disturbances (hypokalemia, hypokalemic metabolic alkalosis, hypernatremia, hypocalcemia); and edema.[43319] [44547] [44557] [51324] In a review of 93 studies of corticosteroid use, hypertension was found to develop approximately 4 times as often in steroid recipients compared to control groups.[24362] [43319] Congestive heart failure can occur in susceptible patients. In a study, an increased risk of heart failure was observed for medium-dose glucocorticoid use as compared with nonuse. At the beginning of the study, patients were at least 40 years of age and had not been hospitalized for cardiovascular disease. Medium exposure was defined as less than 7.5 mg daily of prednisolone or the equivalent given orally, rectally, or parenterally.[30697]
Adverse neurologic effects have been reported during prolonged administration of corticosteroids like prednisone and include amnesia and memory impairment, headache, insomnia, vertigo, restlessness, increased motor activity, impaired cognition, ischemic peripheral neuropathy, neuritis, paresthesias, seizures or convulsions, and EEG changes. Mental disturbances, including depression, anxiety, euphoria, delirium, dementia, hallucinations, irritability, malaise, mania, mood swings, personality changes, schizophrenic reactions, psychosis, and withdrawn behavior, have also been reported; emotional lability and psychotic problems can be exacerbated by corticosteroid therapy.[43319] [51324]
Although corticosteroids like prednisone are used to treat Graves' ophthalmopathy, ocular effects, such as corneal perforation, exophthalmos, posterior subcapsular cataracts, retinopathy, or ocular hypertension, can result from prolonged use of glucocorticoids and could result in glaucoma or ocular nerve damage including optic neuritis. Temporary or permanent visual impairment, including blurred vision and blindness, has been reported with glucocorticoid administration by several routes of administration including intranasal and ophthalmic administration. Secondary fungal and viral ocular infection can be exacerbated by corticosteroid therapy.[43319] [51324]
Prolonged corticosteroid therapy with prednisone may adversely affect the endocrine system, resulting in hypercorticism (Cushing syndrome including fat abnormalities such as buffalo hump and moon face), menstrual irregularity, or decreased carbohydrate and glucose tolerance.[43319] [51324] Systemic corticosteroids are a common cause of drug-induced hyperglycemia. In the hospital setting, there is evidence that more than 50% of the individuals receiving high-dose systemic steroids develop hyperglycemia, with many more having at least 1 episode of hyperglycemia or a mean blood glucose of 140 mg/dL or greater. Long-term use produces metabolic and endocrine effects that include insulin resistance that may lead to new diagnoses of diabetes mellitus (DM) in patients without a history of hyperglycemia or DM prior to corticosteroid use. Glucosuria (glycosuria) and aggravation of existing DM may also occur.[68700]
Adverse GI effects associated with corticosteroid administration such as prednisone include nausea, vomiting, and anorexia with subsequent weight loss. Of note, abrupt cessation of corticosteroids can cause anorexia, nausea, vomiting, and weight loss. Appetite stimulation with weight gain, diarrhea, constipation, abdominal pain and/or distention, hiccups, esophageal ulceration, gastritis, GI perforation, GI bleeding, GI irritation, and pancreatitis have also been reported.[43319] [51324] Although it was once believed that corticosteroids contributed to the development of peptic ulcer disease, in a review of 93 studies of corticosteroid use, the incidence of peptic ulcer disease was not found to be higher in steroid recipients compared to control groups. While most of these studies did not utilize endoscopy, it is unlikely that corticosteroids contribute to the development of peptic ulcer disease.[24362]
Various adverse dermatologic effects reported during corticosteroid therapy include skin atrophy or thin, fragile skin, acne vulgaris, acneiform rash, alopecia or thinning scalp hair, skin hyperpigmentation, skin hypopigmentation, sterile abscess, suppressed reactions to skin tests, diaphoresis, xerosis, facial erythema, striae, petechiae, hirsutism, lupus-like symptoms, ecchymosis and easy bruising, perineal pain and irritation, purpura, rash (unspecified), and telangiectasia. Hypersensitivity reactions of corticosteroid like prednisone may manifest as allergic dermatitis, urticaria, anaphylactoid reactions, and/or angioedema.[43319] [51324]
Pharmacologic doses of corticosteroids like prednisone administered for prolonged periods may result in physiological dependence due to hypothalamic-pituitary-adrenal (HPA) suppression. Exogenous corticosteroids exert negative feedback on the pituitary, inhibiting the secretion of adrenocorticotropin (ACTH) and a decrease in ACTH-mediated synthesis of endogenous corticosteroids and androgens by the adrenal cortex results. The severity of glucocorticoid-induced secondary adrenocortical insufficiency varies among individuals and is dependent upon the dose, frequency, time of administration, and duration of therapy. Administering the drug on alternate days may help to alleviate this adverse effect. Patients with HPA suppression will require increased doses of corticosteroid therapy during periods of physiologic stress. Acute adrenal insufficiency and even death may occur if the sudden withdrawal of the drugs is undertaken. Withdrawal from prolonged oral corticosteroid therapy should be gradual; HPA suppression can last for up to 12 months following cessation of therapy, and patients may need supplemental corticosteroid treatment during periods of physiologic stress, such as surgery, acute blood loss, or infection, even after the drug has been discontinued. Also, a withdrawal syndrome may occur following abrupt discontinuance of corticosteroid therapy and is unrelated to adrenocortical insufficiency. This syndrome includes symptoms such as anorexia, lethargy, nausea/vomiting, headache, fever, arthralgia, myalgia, exfoliative dermatitis, weight loss, and hypotension. These effects are thought to be due to the sudden change in glucocorticoid concentration rather than to low corticosteroid levels. Increased intracranial pressure with papilledema (i.e., pseudotumor cerebri) has also been reported with glucocorticoids usually after treatment.[43319] [51324]
Hypercholesterolemia, atherosclerosis, fat (lipid) embolism, sinus tachycardia, palpitations, bradycardia, syncope, vasculitis, necrotizing angiitis, thrombosis, thromboembolism, and thrombo-phlebitis have been associated with corticosteroid therapy, including prednisone. Glucocorticoid use appears to increase the risk of cardiovascular events such as myocardial infarction, left ventricular rupture (in persons who recently experienced a myocardial infarction), angina, angioplasty, coronary revascularization, stroke, transient ischemic attack, cardiomegaly, arrhythmia exacerbation and ECG changes, hypertrophic cardiomyopathy (in premature infants), and pulmonary edema, cardiac arrest or cardiovascular death. As determined from observational data, the rate of cardiovascular events was 17 per 1,000 person-years among 82,202 non-users of glucocorticoids. In contrast, the rate was 23.9 per 1,000 person-years among 68,781 glucocorticoid users. Furthermore, the rate of cardiovascular events was 76.5 per 1,000 person-years for high exposure patients. After adjustment for known covariates by multivariate analysis, high-dose glucocorticoid use was associated with a 2.56-fold increased risk of cardiovascular events as compared with nonuse. At the beginning of the study, patients were at least 40 years of age and had not been hospitalized for cardiovascular disease. High glucocorticoid exposure was defined as at least 7.5 mg daily of prednisolone, or an equivalent (oral rectal, or parenteral) whereas medium exposure was defined as less than the listed dosage by any of the 3 routes. Low-dose exposure was defined as inhaled, topical, or nasal usage only.[30697] [43319] [51324]
Dizziness and anemia have been reported with corticosteroid use such as prednisone. Corticosteroids may decrease serum concentrations of vitamin C (ascorbic acid) and vitamin A, which may rarely produce symptoms of vitamin A deficiency or vitamin C deficiency. Some loss of folic acid may also be caused by corticosteroid use; glossitis may be noted.[43319] [51324]
Cases of elevated hepatic enzymes (usually reversible upon discontinuation) and hepatomegaly have been associated with prednisone treatment.[43319] [51324]
The coadministration of certain medications may lead to harm and require avoidance or therapy modification; review all drug interactions prior to concomitant use of other medications.
This medication is contraindicated in patients with a history of hypersensitivity to it or any of its components.
The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Many, but not all, oral prednisone products are labeled with this contraindication.[29779][51324]
Corticosteroids, including prednisone, may cause dose-dependent immunosuppression and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids may mask symptoms and increase the risk of severe or fatal infectious complications, requiring close monitoring and potential dose adjustment or withdrawal. Prednisone and other corticosteroids may cause severe or fatal infections by reactivating or worsening TB, hepatitis B, amebiasis, Strongyloides, varicella, measles, herpes simplex, and other viral or herpes infections. Avoid using corticosteroids in individuals with cerebral malaria. Screen for hepatitis B before prolonged immunosuppressive therapy; consult specialists and consider antiviral prophylaxis if infection is present. Rule out latent amebiasis in individuals with tropical exposure or unexplained diarrhea. Use caution in individuals with known or suspected Strongyloides infection; prophylactic therapy may be indicated. Prednisone is generally considered contraindicated for use if a systemic fungal infection is present; many, but not all, oral products are labeled with this contraindication. Corticosteroids, including prednisone, may exacerbate systemic fungal infection; therefore, avoid corticosteroid use in the presence of a fungal infection unless a corticosteroid is needed to control drug reactions. If a fungal infection develops during chronic corticosteroid therapy, corticosteroid withdrawal or dosage reduction is recommended. Corticosteroids should be used with caution, if at all, in individuals with ocular herpes simplex. Avoid exposing nonimmune corticosteroid-treated individuals to varicella or measles. If exposure occurs, prophylaxis with varicella zoster immune globulin (VZIG) for varicella or immunoglobulin (IG) for measles may be needed. If infection develops, consider antiviral treatment.[29779] [43319] [51324]
Corticosteroid therapy, including prednisone therapy, has been associated with left ventricular free-wall rupture in individuals with recent myocardial infarction, and should therefore be used cautiously in these individuals.[29779] [51324]
As sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids, these agents should be used with caution in patients with congestive heart failure, hypertension, renal impairment, or renal failure.[29779] [43319] [51324]
Systemic corticosteroids, such as prednisone, may decrease glucose tolerance, produce hyperglycemia, and aggravate or precipitate diabetes mellitus. When corticosteroid therapy is necessary in individuals with diabetes mellitus, changes in insulin, oral antidiabetic agent dosage, and/or diet may be required.[29779] [43319] [51324]
Metabolic clearance of corticosteroids is decreased in hypothyroidism and increased in hyperthyroidism. Changes in thyroid disease status of an individual may necessitate adjustment in dosage.[29779] [43319] [51324]
Systemic corticosteroids should be used with caution in individuals with active or latent peptic ulcer disease, diverticulitis, fresh intestinal anastomosis, and nonspecific ulcerative colitis, since steroids may increase the risk of a gastrointestinal (GI) perforation. Signs of peritoneal irritation following GI perforation in individuals receiving corticosteroids may be minimal or absent. Corticosteroids should not be used in individuals where there is a possibility of impending GI perforation, GI abscess, or pyogenic infection.[29779] [43319] [51324]
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in individuals with neuromuscular disease disorders (e.g., myasthenia gravis), or in individuals receiving concomitant therapy with neuromuscular blocking drugs. This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.[29779] [43319] [51324]
Systemic corticosteroid use may be associated with neuro-psychiatric effects ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychosis. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids, such as in individuals with a mood disorder or psychotic disorder. Inform individuals or caregivers of the potential for mood and behavioral changes with prednisone treatment and encourage them to seek medical attention if psychiatric symptoms develop, especially if depressed mood (depression) or suicidal ideation is suspected.[29779] [43319] [51324]
If systemic corticosteroids such as prednisone must be used during pregnancy, the potential risks should be discussed with the patient. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism. Based on findings from human and animal studies, corticosteroids can cause fetal harm when administered to a pregnant woman. Published epidemiological studies suggest a small but inconsistent increased risk of orofacial clefts with use of systemic corticosteroids during the first trimester. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring. Intrauterine growth restriction and decreased birth weight have also been reported with maternal use of systemic corticosteroids during pregnancy; however, the underlying maternal condition may also contribute to these risks. There are no adequate and well-controlled studies in pregnant women.[29779] [43319] [51324]
Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (e.g., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to the development of osteopenia and osteoporosis at any age. Use prednisone with caution in individuals with osteoporosis. Special consideration should be given to individuals at increased risk for osteoporosis (e.g., postmenopausal females) before initiating corticosteroid therapy.[29779] [43319] [51324]
Corticosteroids distribute into breast milk, and the manufacturer states that in order to minimize infant exposure, the lowest dose should be prescribed to lactating women to achieve the desired clinical effect.[29779] [43319] [51324] Prednisone concentrations in breast milk are low, and no adverse effects have been reported in the breast-fed infant with maternal use of any corticosteroid during breast-feeding; prednisone is generally considered compatible to use during lactation.[27500] [61288] Published case reports of systemic prednisone use during pregnancy that indicate little risk to a nursing infant due to lack of reported side effects. Prednisone is converted to prednisolone in vivo, and peak concentrations in human milk appear in about 1 hour after a dose; the total daily dose reaching the infant is approximately 0.1% of the mother's total daily dose.[49754] Prednisolone and methylprednisolone have similar data available regarding systemic use during lactation. High doses of corticosteroids administered to lactating women for long periods could potentially produce problems in the breastfed infant including growth and development and interfere with endogenous corticosteroid production.[51324] At higher daily prednisone doses, avoidance of breast-feeding during times of peak milk concentrations can help limit infant exposure; however, such adjustments are rarely necessary.[61288] Consider the benefits of breast-feeding, the risk of potential infant drug exposure, and the risk of an untreated or inadequately treated condition.
Use systemic corticosteroids such as prednisone with caution in the geriatric adult; the risks and benefits of therapy should be considered for any individual patient, particularly with chronic use. According to the Beers Criteria, systemic corticosteroids are considered potentially inappropriate medications (PIMs) for use in geriatric patients with delirium or at high risk for delirium; avoid when possible in these patient populations due to the possibility of new-onset delirium or exacerbation of the current condition. Oral and parenteral corticosteroids may be required for conditions such as exacerbation of chronic obstructive pulmonary disease (COPD) but should be prescribed in the lowest effective dose and for the shortest possible duration.[63923]
Individuals with hepatic cirrhosis can have an exaggerated response to systemic corticosteroids, such as prednisone.[29779] [43319]
Glucocorticoids are naturally occurring hormones that prevent or suppress inflammation and immune responses when administered at pharmacological doses. At a molecular level, unbound glucocorticoids readily cross cell membranes and bind with high affinity to specific cytoplasmic receptors. This binding induces a response by modifying transcription and, ultimately protein synthesis to achieve the steroid's intended action. Such actions may include: inhibition of leukocyte infiltration at the site of inflammation, interference in the function of mediators of inflammatory response, and suppression of humoral immune responses. Some of the net effects include reduction in edema or scar tissue, as well as a general suppression in immune response. The degree of clinical effect is normally related to the dose administered. The antiinflammatory actions of corticosteroids are thought to involve phospholipase A2 inhibitory proteins, collectively called lipocortins. Lipocortins, in turn, control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of the precursor molecule arachidonic acid. Likewise, the numerous adverse effects related to corticosteroid use are usually related to the dose administered and the duration of therapy.[30943][50600]
Revision Date: 10/06/2025, 03:06:16 PMPrednisone is administered orally as immediate-release (IR) tablets, an oral solution, or delayed-release tablets. Prednisone binds extensively to the plasma proteins albumin and transcortin, with only the unbound portion of a dose active. Systemic prednisone is quickly distributed into the kidneys, intestines, skin, liver, and muscle. Corticosteroids distribute into the breast milk and cross the placenta. Prednisone is metabolized by the liver to the active metabolite prednisolone, which has a 4- to 6-fold higher exposure than that of prednisone. Prednisolone is formed through the 11b-hydroxydehydrogenase enzyme, which is not part of the CYP system, but then prednisolone is metabolized by the CYP3A4-mediated 6b-hydroxylase enzyme to inactive compounds. These inactive metabolites, as well as a small portion of unchanged drug, are excreted in the urine. The plasma elimination half-life is 1 hour, whereas the biological half-life of prednisone is 18 to 36 hours. After oral administration of the delayed-release tablets, the terminal half-life of both prednisone and prednisolone was 2 to 3 hours, which is comparable to that from the IR formulation.[51324]
Affected cytochrome P450 isoenzymes and drug transporters: P-gp, CYP3A4
In vitro, prednisone was identified as a substrate of p-glycoprotein (P-gp).[34354] Prednisone is metabolized by the liver to the active metabolite prednisolone via the 11b-hydroxydehydrogenase enzyme, which is not part of the CYP system, but prednisolone is metabolized by the CYP3A4-mediated 6b-hydroxylase enzyme to inactive compounds.
There is an enhanced effect of corticosteroids in patients with cirrhosis.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function.
If systemic corticosteroids such as prednisone must be used during pregnancy, the potential risks should be discussed with the patient. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism. Based on findings from human and animal studies, corticosteroids can cause fetal harm when administered to a pregnant woman. Published epidemiological studies suggest a small but inconsistent increased risk of orofacial clefts with use of systemic corticosteroids during the first trimester. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring. Intrauterine growth restriction and decreased birth weight have also been reported with maternal use of systemic corticosteroids during pregnancy; however, the underlying maternal condition may also contribute to these risks. There are no adequate and well-controlled studies in pregnant women.[29779] [43319] [51324]
Corticosteroids distribute into breast milk, and the manufacturer states that in order to minimize infant exposure, the lowest dose should be prescribed to lactating women to achieve the desired clinical effect.[29779] [43319] [51324] Prednisone concentrations in breast milk are low, and no adverse effects have been reported in the breast-fed infant with maternal use of any corticosteroid during breast-feeding; prednisone is generally considered compatible to use during lactation.[27500] [61288] Published case reports of systemic prednisone use during pregnancy that indicate little risk to a nursing infant due to lack of reported side effects. Prednisone is converted to prednisolone in vivo, and peak concentrations in human milk appear in about 1 hour after a dose; the total daily dose reaching the infant is approximately 0.1% of the mother's total daily dose.[49754] Prednisolone and methylprednisolone have similar data available regarding systemic use during lactation. High doses of corticosteroids administered to lactating women for long periods could potentially produce problems in the breastfed infant including growth and development and interfere with endogenous corticosteroid production.[51324] At higher daily prednisone doses, avoidance of breast-feeding during times of peak milk concentrations can help limit infant exposure; however, such adjustments are rarely necessary.[61288] Consider the benefits of breast-feeding, the risk of potential infant drug exposure, and the risk of an untreated or inadequately treated condition.
Cookies are used by this site. To decline or learn more, visit our cookie notice.
Copyright © 2025 Elsevier, its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.