ThisiscontentfromElsevier'sDrugInformation
SARS-CoV-2 Virus (COVID-19) mRNA Vaccine
Learn more about Elsevier's Drug Information today! Get the drug data and decision support you need, including TRUE Daily Updates™ including every day including weekends and holidays.
General Dosing Information
0.3 mL (30 mcg) IM.[67786] Alternatively, 0.3 mL (30 mcg) IM for 2 doses. Administer the second dose 6 months (minimum interval 2 months) after dose 1.[53026]
0.3 mL (30 mcg) IM.[53026]
0.3 mL (30 mcg) IM.[53026] [72585]
0.3 mL (10 mcg) IM.[53026] [72585]
0.5 mL (50 mcg) IM.[67339] Alternatively, 0.5 mL (50 mcg) IM for 2 doses. Administer the second dose 6 months (minimum interval 2 months) after dose 1.[53026]
0.5 mL (50 mcg) IM.[53026]
0.5 mL (50 mcg) IM.[53026] [72585]
0.25 mL (25 mcg) IM.[53026] [72585]
0.25 mL (25 mcg) IM for 2 doses. Administer dose 1 at week 0 and dose 2 at week 4 to 8.[53026] Alternatively, 0.25 mL (25 mcg) IM as a single dose.[72585]
0.25 mL (25 mcg) IM for 2 doses. Administer dose 1 at week 0 and dose 2 at week 4 to 8.[53026] [72585]
0.2 mL (10 mcg) IM once.[72298]
0.3 mL (30 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[67786] Alternatively, 0.3 mL (30 mcg) IM for 2 doses. Administer first updated dose at least 8 weeks after the last dose of COVID-19 vaccine and second dose 6 months (minimum interval 2 months) after dose 1.[53026]
0.3 mL (30 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026]
0.3 mL (30 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.3 mL (10 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.5 mL (50 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[67339] Alternatively, 0.5 mL (50 mcg) IM for 2 doses. Administer first updated dose at least 8 weeks after the last dose of COVID-19 vaccine and second dose 6 months (minimum interval 2 months) after dose 1.[53026]
0.5 mL (50 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026]
0.5 mL (50 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.25 mL (25 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.25 mL (25 mcg) IM 4 to 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.25 mL (25 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.25 mL (25 mcg) IM 4 to 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.25 mL (25 mcg) IM at least 8 weeks after the last dose of COVID-19 vaccine.[53026] [72585]
0.2 mL (10 mcg) IM once at least 3 months after the last dose of COVID-19 vaccine.[72298]
0.3 mL (30 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose 8 weeks after the last dose.[67786] Alternatively, 0.3 mL (30 mcg) IM for 2 doses. Administer the second dose 6 months (minimum interval 2 months) after dose 1.[53026]
0.3 mL (30 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose 8 weeks after the last dose.[67786]
0.3 mL (30 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose 8 weeks after the last dose.[67786] [72585]
0.3 mL (10 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose 8 weeks after the last dose.[67786] [72585]
0.5 mL (50 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose at least 8 weeks after the last dose.[67339] Alternatively, 0.5 mL (50 mcg) IM for 2 doses. Administer the second dose 6 months (minimum interval 2 months) after dose 1.[53026]
0.5 mL (50 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose 8 weeks after the last dose.[67339]
0.5 mL (50 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose at least 8 weeks after the last dose.[67339] [72585]
0.25 mL (25 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose at least 8 weeks after the last dose of COVID-19 vaccine.[67339] [72585]
0.25 mL (25 mcg) IM for 2 doses. Administer dose 1 at week 0 and dose 2 between weeks 4 to 8.[67339] [72585]
0.25 mL (25 mcg) IM once 4 to 8 weeks after the last dose of Moderna COVID-19 vaccine.[67339] [72585]
0.25 mL (25 mcg) IM once at least 8 weeks after the last dose of Moderna COVID-19 vaccine.[67339]
0.2 mL (10 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose at least 3 months after the last dose.[72298]
0.2 mL (10 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose at least 3 months after the last dose.[72298]
0.2 mL (10 mcg) IM once. If previously vaccinated with any COVID-19 vaccine, administer the updated vaccine dose at least 3 months after the last dose.[72298] [72585]
0.3 mL (30 mcg) IM for 4 doses. Administer the second dose 3 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.3 mL (30 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 3 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.3 mL (30 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.3 mL (30 mcg) IM for 4 doses. Administer the second dose 3 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.3 mL (30 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 3 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.3 mL (30 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.3 mL (10 mcg) IM for 4 doses. Administer the second dose 3 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.3 mL (10 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 3 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.3 mL (10 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.5 mL (50 mcg) IM for 4 doses. Administer the second dose 4 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.5 mL (50 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 4 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.5 mL (50 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026]
0.5 mL (50 mcg) IM for 4 doses. Administer the second dose 4 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.5 mL (50 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 4 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.5 mL (50 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.25 mL (25 mcg) IM for 4 doses. Administer the second dose 4 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.25 mL (25 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 4 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.25 mL (25 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. In children turning from 11 to 12 years during the initial vaccination series, complete the series using the dose for 12 years and older for all doses received on or after turning 12 years. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.25 mL (25 mcg) IM for 4 doses. Administer the second dose 4 weeks after dose 1, the third dose at least 4 weeks after dose 2, and the fourth dose 6 months (minimum interval 2 months) after dose 3. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.25 mL (25 mcg) IM for 2 to 3 doses. If previously vaccinated with 1 dose of COVID-19 vaccine, administer 3 updated doses. Administer the first dose 4 weeks after last dose, the second dose at least 4 weeks after dose 1, and the third dose 6 months (minimum interval 2 months) after dose 2. If previously vaccinated with 2 doses of COVID-19 vaccine, administer 2 updated doses. Administer the first dose at least 4 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.25 mL (25 mcg) IM for 2 doses. Administer the first dose at least 8 weeks after last dose and the second dose 6 months (minimum interval 2 months) after dose 1. Further additional doses may be administered at least 2 months after the last dose at the discretion of the health care provider based on the patient's clinical circumstances.[53026] [72585]
0.2 mL (10 mcg) IM at least 3 months after the last dose of COVID-19 vaccine.[72298]
0.2 mL (10 mcg) IM at least 3 months after the last dose of COVID-19 vaccine.[72298]
0.2 mL (10 mcg) IM at least 3 months after the last dose of COVID-19 vaccine.[72298]
0.3 mL/dose (30 mcg) IM for Pfizer-BioNTech COVID-19 vaccine; 0.5 mL/dose (50 mcg) IM for Moderna COVID-19 vaccine; 0.2 mL/dose (10 mcg) IM for mNexspike.
0.3 mL/dose (30 mcg) IM for Pfizer-BioNTech COVID-19 vaccine; 0.5 mL/dose (50 mcg) IM for Moderna COVID-19 vaccine; 0.2 mL/dose (10 mcg) IM for mNexspike.
0.3 mL/dose (30 mcg) IM for Pfizer-BioNTech COVID-19 vaccine; 0.5 mL/dose (50 mcg) IM for Moderna COVID-19 vaccine; 0.2 mL/dose (10 mcg) IM for mNexspike.
12 years: 0.3 mL/dose (30 mcg) IM for Pfizer-BioNTech COVID-19 vaccine; 0.5 mL/dose (50 mcg) IM for Moderna COVID-19 vaccine; 0.2 mL/dose (10 mcg) IM for mNexspike.
5 to 11 years: 0.3 mL/dose (10 mcg) IM for Pfizer-BioNTech COVID-19 vaccine; 0.25 mL/dose (25 mcg) IM for Moderna COVID-19 vaccine; safety and efficacy have not been established for mNexspike.
1 to 4 years: 0.25 mL/dose (25 mcg) IM for Moderna COVID-19 vaccine; safety and efficacy have not been established for Pfizer-BioNTech COVID-19 vaccine or mNexspike.
6 to 11 months: 0.25 mL/dose (25 mcg) IM for Moderna COVID-19 vaccine; safety and efficacy have not been established for Pfizer-BioNTech COVID-19 vaccine or mNexspike.
1 to 5 months: Safety and efficacy have not been established.
Safety and efficacy have not been established.
Specific guidelines for dosage adjustments in hepatic impairment are not available; it appears that no dosage adjustments are needed.
Specific guidelines for dosage adjustments in renal impairment are not available; it appears that no dosage adjustments are needed.
† Off-label indication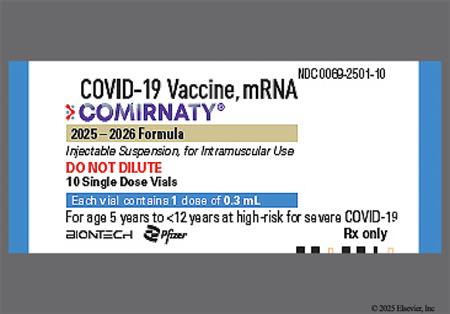
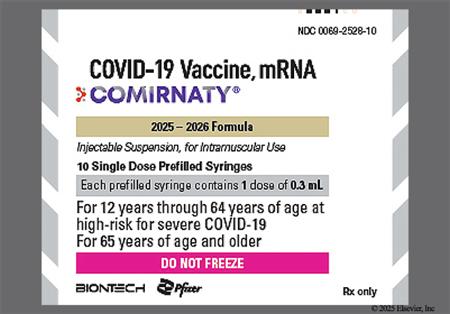

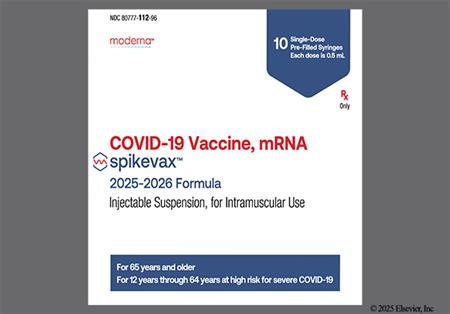

NOTE: The 2025/2026 COVID-19 vaccine strain targets the Omicron variant sublineage LP.8.1.[67339][67786]
The COVID-19 vaccine is a vaccine that contains messenger RNA (mRNA) encoding the viral spike glycoprotein (S) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is used for active immunization for the prevention of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2. One of the advantages of mRNA vaccines is that they can be rapidly manufactured. The process is cell-free and can be scaled, allowing quick responses to large outbreaks and epidemics, such as the COVID-19 pandemic. Additionally, mRNA vaccines offer a different technology; unlike other vaccines, RNA-based vaccines introduce an mRNA sequence coded for a disease-specific antigen, which elicits a robust innate immune response when presented to the immune system.[66084][66093] Myocarditis and pericarditis have been reported with use of mRNA vaccines. The highest risk appears to be in males aged 12 to 24 years. Based on claims data following the administration of the 2023-2024 formulation of mRNA COVID-19 vaccines, there were approximately 8 cases per million in individuals 6 months to 64 years of age and approximately 27 cases per million doses in males 12 to 24 years of age. Persistence of abnormal cardiac magnetic resonance imaging (CMR) findings, which is a marker for myocardial injury, has been found in individuals with COVID-19 vaccine-associated myocarditis. The clinical and prognostic significance of these CMR findings are not known. The most commonly reported adverse drug reactions include injection site pain, fever, chills, fatigue, muscle pain, and headache. Serious allergic reactions and anaphylaxis have been reported in patients outside of clinical trials during mass vaccination. The vaccines Comirnaty (Pfizer), Spikevax (Moderna), and low dose mNexspike were FDA-approved in 2021, 2022, and May 2025, respectively.[66084][67339][67786][68453][72298]
For storage information, see the specific product information within the How Supplied section.
Storage and Handling Prior to Thawing (Comirnaty single-dose vials)
Storage and Handling Prior to Thawing (Spikevax prefilled syringes)
Storage and Handling Prior to Thawing (mNexspike prefilled syringes)
Comirnaty COVID-19 vaccine
Thawing
Intramuscular Injection
Prefilled Syringe
Vial
Spikevax COVID-19 vaccine
Thawing
Preparation
Intramuscular Injection
mNexspike COVID-19 vaccine
Thawing
Preparation
Intramuscular Injection
Injection site reaction is commonly reported after COVID-19 vaccination. In patients aged 12 years and older receiving Pfizer-BioNTech COVID-19 vaccination during clinical trials, injection site pain (57.1% to 90.5%; less than 2% severe), injection site swelling (2.7% to 11.8%; less than 1% severe), and injection site redness (3.7% to 10.4%; less than 1% severe) were reported. The mean duration of pain at the injection site was 2.1 to 2.5 days (range: 1 to 70 days), for redness 1.5 to 3 days (range: 1 to 34 days), and swelling 1.3 to 2.6 days (range: 1 to 34 days). In patients aged 5 to 11 years receiving the Pfizer-BioNTech COVID-19 vaccine, injection site pain or tenderness (71.2% to 83.8%; less than 1% severe), swelling (10.3% to 20%; less than 1% severe), and redness (14% to 25.9%; less than 1% severe) were reported. The mean duration of pain at the injection site was 2.3 days (range: 1 to 37 days), for redness 2 days (range: 1 to 10 days), and swelling 2.2 days (range: 1 to 16 days).[67786] In adult individuals receiving Moderna COVID-19 vaccination or mNexspike vaccine during clinical trials, pain (54.6% to 86.3%; 0.7% to 5% Grade 3), axillary swelling/tenderness (5% to 25%; less than 2% Grade 3), swelling (2.6% to 13%; less than 2% Grade 3), erythema (2% to 9%; less than 2% Grade 3), and rash (2%) were reported. Grade 3 local adverse reactions were more frequently reported after Dose 2 than after Dose 1. Injection site rash or urticaria, likely related to the vaccination, were reported in 6 adults vs. 0 individuals receiving placebo. The median duration of adverse reactions was 2 to 3 days. Delayed injection site reactions (occurring more than 7 days after administration), such as pain, erythema, and swelling were reported in 1.4% of adult individuals. In pediatric individuals (age: 6 months to 17 years) who received Moderna COVID-19 vaccine or mNexspike vaccine during clinical trials, pain (11.1% to 98.4%; 6% or less Grade 3), axillary swelling/tenderness (2.6% to 39.5%; 1.5% or less Grade 3), swelling (1.3% to 22.3%; 0% to 3% Grade 3), erythema (1.2% to 24.1%; less than 1% to 3% Grade 3), and injection site rash or urticaria (0.3%) were reported. The median duration of adverse reactions was 1 to 4 days. Delayed injection site reactions (occurring more than 7 days after administration), such as pain, erythema, and swelling were reported in 1.5% of pediatric individuals.[67339] [72298]
In individuals 16 years and older receiving Pfizer-BioNTech COVID-19 vaccination, fatigue (33.7% to 61.5%; 0.1% to 5.3% severe), and headache (25% to 54%; 3.4% or less severe), were reported during clinical trials. Malaise, asthenia, hyperhidrosis, lethargy, and night sweats were unsolicited adverse events reported in less than 1% of individuals 16 years and older. In individuals 12 to 15 years, fatigue was reported in 60.1% to 66.2% (1.3% to 2.4% severe) and headache was reported in 55.3% to 64.5% (1% to 2% severe). In pediatric individuals (age: 5 to 11 years) who received the Pfizer-BioNTech COVID-19 vaccination, fatigue (up to 51.9%; less than 1% severe) and headache (up to 38.4%; less than 1% severe) were reported. Syncope and dizziness have been reported during postmarketing experience with Pfizer-BioNTech COVID-19 vaccine.[67786] In adult individuals receiving Moderna COVID-19 vaccine or mNexspike vaccine, fatigue (33.3% to 67.8%; 0.8% to 10.7% Grade 3) and headache (24.5% to 63%; 0.5% to 5.1% Grade 3) were reported during clinical trials. In pediatric individuals (age: 37 months to 17 years) receiving Moderna COVID-19 vaccine or mNexspike vaccine, fatigue (12.2% to 73.2%; 1% to 8% Grade 3) and headache (11.5% to 70.5%; 0.2% to 7.5% Grade 3) were reported during clinical trials. Syncope has been reported during postmarketing experience with Moderna COVID-19 vaccine. Prior to administration, ensure procedures are in place to prevent falls and manage syncopal reactions. Patients should remain seated or lying down during the observation period to decrease the risk for injury. If syncope develops, observe patients until symptoms resolve.[66175] [67339] [67786] [72298]
In patients receiving Pfizer-BioNTech COVID-19 vaccine, new or worsened myalgia (13.6% to 46%; 2.3% or less severe) and new or worsened arthralgia (8.7% to 28%; 1% or less severe) were reported in patients (age: 12 years or older) during clinical trials. New or worsened myalgia (9.3% to 18.1%; less than 1% severe) and new or worsened arthralgia (3.4% to 7.6%; 0 severe) were reported in pediatric patients (age: 5 to 11 years) receiving Pfizer-BioNTech COVID-19 vaccine during clinical trials. Pain in the extremity (arm) has been reported during postmarketing experience with Pfizer-BioNTech COVID-19 vaccination.[67786] In adult patients receiving Moderna COVID-19 vaccination or mNexspike vaccine, myalgia (19.7% to 61.7%; 0.5% to 10.1% Grade 3) and arthralgia (16.4% to 45.6%; less than 5.9% Grade 3) were reported in clinical trials. Myalgia (15.6% to 47.1%; 0.3% to 5.6% Grade 3) and arthralgia (9.8% to 29.3%; less than 2.4% Grade 3) were reported in pediatric patients (age: 12 to 17 years) receiving Moderna COVID-19 vaccination or mNexspike vaccine during clinical trials. Myalgia (9.9% to 35.3%; 2.4% or less Grade 3) and arthralgia (6.2% to 21.3%; less than 1% Grade 3) were reported in pediatric patients (age: 37 months to 11 years) receiving Moderna COVID-19 vaccination during clinical trials.[67339] [72298]
Chills (6.5% to 37.8%; less than 3% severe) and fever (1.3% to 18%) were reported in patients (age: 16 years and older) receiving Pfizer-BioNTech COVID-19 vaccination during clinical trials. In patients (age: 12 years and older) receiving a booster dose with Pfizer-BioNTech COVID-19 vaccine, bivalent the following were reported: chills (12 to 23.4%) and fever (4.7% to 9.3%). Chills (27.6% to 49.2%; less than 2% severe) and fever (10.1% to 24.3%) were reported in pediatric patients (age: 12 to 15 years) who received the Pfizer-BioNTech COVID-19 vaccination during clinical trials. Chills (5.6% to 13.3%; less than 1% severe) and fever (2.1% to 7.8%) were reported in patients (age: 5 to 11 years) who received the Pfizer-BioNTech COVID-19 vaccine. Febrile seizures have been reported during postmarketing experience with Pfizer-BioNTech COVID-19 vaccine.[67786] Chills (5.3% to 48.7%; less than 2% Grade 3) and fever (less than 1% to 17.4%; less than 2% Grade 3) were reported in adult individuals receiving Moderna COVID-19 vaccine or mNexspike vaccine during clinical trials. Chills (5.3% to 49%; 1.2% or less Grade 3) and fever (1.3% to 24%; less than 4% Grade 3) were reported in pediatric individuals (age: 6 to 17 years) receiving Moderna COVID-19 vaccine or mNexspike vaccine during clinical trials. Chills (5.8% to 12.3%; less than 1% Grade 3) and fever (7.6% to 15.8%; less than 3% Grade 3) were reported in pediatric individuals (age: 37 months to 5 years). Fever was reported in up to 14.2% of pediatric individuals (age: 6 months to 23 months) receiving Moderna COVID-19 vaccination during clinical trials. A 1-year-old female had a Grade 3 fever 6 hours after Dose 1 and febrile convulsions 1 day after Dose 1. Febrile seizures have been reported during postmarketing experience with Moderna COVID-19 vaccine.[67339] [72298]
In individuals (age: 12 years and older) receiving the Pfizer-BioNTech COVID-19 vaccine, vomiting (0.5% to 2.8%; less than 1% severe) and diarrhea (5.9% to 10.7%; less than 1% severe) were reported during clinical trials. In individuals (age: 16 and older) who received the Pfizer-BioNTech COVID-19 vaccine, nausea was reported in 274 individuals receiving the vaccine vs. 87 individuals who received placebo and decreased appetite was reported in 39 individuals receiving the vaccine vs. 9 individuals who received placebo. Nausea (0.3% to 0.7%) and decreased appetite (0.3%) were reported in adult individuals and nausea was reported in 5 pediatric individuals vs. 2 individuals receiving placebo (age: 12 to 15). Vomiting (2%), diarrhea (5.4% to 6.4%), nausea (0.2%) and decreased appetite (0.1% to 0.3%) were reported in individuals aged 5 to 11 years.[67786] In adult individuals receiving the Moderna COVID-19 vaccine, nausea/vomiting (4% to 21%; less than 2% Grade 3) was reported during clinical trials. Nausea/vomiting (5% to 29.3%; less than 1% Grade 3) was reported in pediatric individuals (age: 37 months to 17 years) receiving Moderna COVID-19 vaccine during clinical trials. Loss of appetite/anorexia (up to 46.5%) was reported in pediatric individuals (age: 6 to 36 months) receiving Moderna COVID-19 vaccination.[67339]
Myocarditis and pericarditis have been reported during postmarketing experience with mRNA vaccines. The observed risk is highest in males 12 to 24 years within 7 days after vaccination, occurring more often after the second dose. However, cases have also been observed in females and after other doses. Symptoms include chest pain, shortness of breath, or palpitations; younger children may have more non-specific symptoms such as irritability, vomiting, poor feeding, tachypnea, or lethargy. Most patients require hospitalization but experience resolution of acute symptoms; long-term effects are unknown. In a population-based cohort study of 3,095,414 people aged 18 years and older, a 2 to 3-fold higher incidence of myocarditis/pericarditis was observed in people who received a second mRNA vaccination with the Moderna COVID-19 vaccine compared to the Pfizer-BioNTech vaccine. The higher incidence of myocarditis after the Moderna vaccine was highest among younger men (18 to 39 years) and was not present at older ages (40 years and older). However, the odds of pericarditis were higher with the Moderna vaccine for both younger and older individuals. In a retrospective observational cohort examining cardiovascular outcomes in 307 individuals (age: 5 to 29 years) diagnosed with myocarditis linked to mRNA COVID-19 vaccination, at a median follow-up of about 3 months, 89 individuals reported ongoing cardiac symptoms. Cardiac magnetic resonance imaging (CMR) was performed on 216 patients. Overall, 177 of these had late gadolinium enhancement (LGE), a marker of myocardial injury. Among 161 individuals who had LGE on initial CMR and had a follow-up gadolinium-enhanced CMR at a median of 159 days, 98 individuals had persistent LGE. Overall, the severity of LGE decreased during follow-up. The clinical significance of these MRI findings remains unclear. Development of myocarditis or pericarditis within 3 weeks of COVID-19 vaccination is a precaution against subsequent doses of any COVID-19 vaccine, and subsequent doses should generally be avoided. If the decision is made to receive a subsequent dose, the episode of myocarditis or pericarditis should be resolved. Considerations for subsequent vaccination include myocarditis or pericarditis considered unrelated to mRNA COVID-19 vaccination (e.g., due to SARS-CoV-2 or other viruses, diagnosis was made more than 3 weeks after vaccination), personal risk of severe acute COVID-19 (e.g., age, underlying conditions), or timing of any immunomodulatory therapies. Patients with a history of myocarditis or pericarditis prior to COVID-19 vaccination or more than 3 weeks after a COVID-19 vaccine dose should not receive a mRNA vaccine dose until their episode of myocarditis or pericarditis has completely resolved, which includes no evidence of ongoing heart inflammation or sequelae. Individuals who have a history of other heart disease, including congenital heart disease and Kawasaki disease, may receive any currently FDA-approved COVID-19 vaccine.[66175] [66698] [66770] [67339] [67786] [71812] [72298]
Anaphylactoid reactions, such as anaphylaxis, rash, pruritus, urticaria, and angioedema, have been reported during postmarketing experience with Pfizer-BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine.[67339] [67786] Angioedema/facial swelling was reported in 3 adults receiving the Moderna COVID-19 vaccine during clinical trials. All 3 individuals have a history of injection of dermatological fillers. The onset of swelling was reported 1 to 2 days after the second dose. One serious adverse event of erythema nodosum occurred 8 days after the Moderna COVID-19 booster dose in a 73-year-old female. The event was considered causally related to the Moderna COVID-19 vaccine and was reported as resolved without treatment on Day 30. During a 7-day follow-up period, hypersensitivity adverse reactions (injection site rash, injection site urticaria) were reported in 6 adult individuals receiving Moderna COVID-19 vaccine and none in the placebo group. In pediatric individuals, hypersensitivity adverse reactions (injection site rash, injection site urticaria) were reported in 0.3% of individuals receiving Moderna COVID-19 vaccine and less than 0.1% in the placebo group during a 28-day follow-up period. Delayed injection site reactions that began more than 7 days after vaccination were reported in 1.4% to 1.5% of patients. Delayed reactions included pain, erythema, and swelling.[67339] Appropriate medication treatment must be immediately available to manage potential anaphylactic reactions.[72298]
Lymphadenopathy was reported in 0.4% to 0.9% of individuals (age: 5 years and older) receiving the Pfizer-BioNTech COVID-19 vaccine during clinical trials. In patients receiving a Pfizer-BioNTech COVID-19 vaccine booster dose, lymphadenopathy occurred in 2.8% to 5.2% of individuals aged 16 years and older, 1% of individuals aged 12 to 15 years, and 1.9% of individuals aged 5 to 11 years.[67786] During a 28-day follow-up period after any dose of Moderna COVID-19 vaccine in clinical trials, lymphadenopathy-related events were reported in 1.7% of adults and 6% of pediatric patients (age: 12 to 17 years). These events included lymphadenopathy, lymphadenitis, lymph node pain, vaccination-site lymphadenopathy, injection-site lymphadenopathy, and axillary mass.[67339]
During the Pfizer-BioNTech COVID-19 vaccine safety follow-up period, Bell's palsy (facial paralysis) was reported by 4 individuals. The onset of facial paralysis was Day 37 after Dose 1 (individual did not receive Dose 2), Day 3, 9, and 48 after Dose 2. There were 2 cases in the placebo group, occurring on Day 32 and Day 102. The information available is insufficient to determine a causal relationship with the vaccine.[67786] During Moderna COVID-19 clinical trials, facial paralysis (including Bell's palsy) was reported during the blinded portion in 8 adult patients receiving Moderna COVID-19 vaccination vs. 3 in the placebo group. In the 28-day follow-up period there were 2 cases of facial paralysis, which occurred on Day 8 and Day 22 after vaccination, and 1 case in the placebo group on Day 17 after vaccination. Herpes zoster was reported in 50 adult patients receiving Moderna COVID-19 vaccination during clinical trials vs. 23 in the placebo group. In the 28-day follow-up period there were 22 cases of herpes zoster in those receiving Moderna COVID-19 vaccination vs. 15 in the placebo group. Currently, available information on facial paralysis and herpes zoster infection is insufficient to determine a causal relationship with the vaccine.[67339]
Menstrual irregularity has been reported after mRNA COVID-19 vaccination. A retrospective cohort analysis of prospectively collected data was performed in 19,622 patients (14,936 in the vaccinated group; 4,686 in the unvaccinated group). The study found that when 1 vaccine dose was administered per cycle, there was a 0.71 day increase (99.3% CI; 0.47 to 0.96) in cycle length after the first dose and a 0.56 day increase (99.3% CI; 0.28 to 0.84) after the second dose, compared to unvaccinated patients. The difference was greatest among patients who received 2 vaccine doses in a cycle, with an increase of 3.7 days (99.3% CI; 2.98 to 4.42). Menses length was unaffected by vaccination.[68037]
In January 2023, the Centers for Disease Control (CDC) and the Food and Drug Administration (FDA) identified a potential risk of ischemic stroke in patients 65 years and older who received the Pfizer-BioNTech COVID-19 bivalent vaccine. The CDC's Vaccine Safety Datalink (VSD), a real-time surveillance system, met the statistical criteria to prompt additional investigation into whether patients 65 and older who received the Pfizer-BioNTech COVID-19 bivalent vaccine were more likely to experience an ischemic stroke in the 21 days after vaccination compared with days 22 to 44 days after vaccination. A potential risk was not identified with the Moderna COVID-19 bivalent vaccine. No other safety systems have shown a similar risk and multiple subsequent analyses have not validated this risk. The CDC and FDA will continue to evaluate additional data from these and other vaccine safety systems; however, no change in vaccination practice is recommended.[68453]
Irritability/crying (up to 82.8%) and drowsiness/sleepiness (up to 52.2%) were reported in pediatric patients (age: 6 to 36 months) receiving Moderna COVID-19 vaccine during clinical trials.[67339]
The use of the COVID-19 vaccine has resulted in laboratory test interference with the Bio-Rad Laboratories BioPlex 2200 Syphilis Total and Rapid Plasma Reagin (RPR) kit. False reactivity may occur for at least 5 months after COVID-19 vaccination. It is unknown if other RPR tests may also be affected. Treponemal testing for syphilis such as Treponema pallidum particle agglutination (TP-PA) and treponemal immunoassays do not appear to be impacted. Retest patients who receive a positive result using the Bio-Rad BioPlex 2200 Syphilis Total and RPR kit for syphilis with another test to confirm results. For patients with a negative treponemal test, but reactive RPR result using the Bio-Rad BioPlex 2200 Syphilis Total and RPR kit, repeat RPR testing is not necessary unless otherwise clinically indicated. For patients previously treated for syphilis, whose treponemal testing will remain persistently positive, and are being evaluated for a possible new syphilis infection, interpret a reactive RPR in the context of the patient's medical history, risk factors, and clinical presentation. If the Bio-Rad BioPlex 2200 Syphilis Total and RPR kit was used and the clinical presentation does not support syphilis reinfection, then confirm reactive results with a RPR test from another manufacturer.[67201]
The coadministration of certain medications may lead to harm and require avoidance or therapy modification; review all drug interactions prior to concomitant use of other medications.
This medication is contraindicated in patients with a history of hypersensitivity to it or any of its components. A history of a severe allergic reaction (e.g., anaphylaxis) to a component of a COVID-19 vaccine or to a prior dose is a contraindication to further doses of the same vaccine type. These patients may be considered for an alternative COVID-19 vaccine, if appropriate. Referral to an allergist-immunologist is recommended to evaluate the initial reaction and determine whether re-administration of the original vaccine may be considered based on risk assessment.[66175][67339][67786][72298]
Postponing vaccination with the COVID-19 vaccine is recommended in patients with a moderate or severe acute illness or infection, with or without fever. Defer COVID-19 vaccination until the illness has improved. Advise individuals with known current SARS-CoV-2 infection to defer any COVID-19 vaccination at least until recovery from the acute illness and criteria to discontinue isolation have been met. Individuals who recently had SARS-CoV-2 infection may consider delaying a COVID-19 vaccine dose by 3 months from symptom onset or positive test. Increased time between infection and vaccination may result in an improved immune response to vaccination. Additionally, a low risk of reinfection has been observed in the weeks to months after infection. Take into account risks of COVID-19 severe disease or characteristics of the predominant SARS-CoV-2 strain when determining whether to delay a COVID-19 vaccination after infection.[66175]
Immunocompromised individuals, including individuals with immunosuppression or receiving immunosuppressive therapy, may not have an adequate immune response to the COVID-19 vaccine.[67339] [67786] [72298] Immunosuppressed persons may include individuals with congential or acquired immunodeficiencies, whether due to concurrent disease (e.g., HIV infection, leukemia, lymphoma), cancer therapy (e.g., cytotoxic drugs, radiation), or immunosupressive therapy (e.g., corticosteroids). Short-term (less than 2 weeks) corticosteroid therapy or intra-articular, bursal, or tendon injections with corticosteroids should not be immunosuppressive. COVID-19 vaccines may be administered without regard to timing of corticosteroid treatment, including topical or intra-articular treatment, bursal, or tendon injection.[65107] [66175] Ideally, complete COVID-19 vaccination at least 2 weeks before initiation or resumption of immunosuppressive therapies, but timing of COVID-19 vaccination should take into consideration current or planned immunosuppressive therapies and optimization of both the individual's medical condition and response to vaccine. Serologic testing or cellular immune testing to assess for immunity after COVID-19 vaccination, outside the context of research studies, is not recommended.[66175]
Data suggest immune response to COVID-19 vaccination may be reduced in patients with renal failure receiving dialysis. Counsel dialysis patients about the potential for reduced immune responses and the need to continue following precautions to avoid exposure to the SARS-CoV-2 virus.[69469]
Myocarditis and pericarditis have been reported during postmarketing experience with mRNA vaccines. Development of myocarditis or pericarditis within 3 weeks of COVID-19 vaccination is a precaution for subsequent doses of any COVID-19 vaccine, and subsequent doses should generally be avoided. If the decision is made to receive a subsequent dose, the episode of myocarditis or pericarditis should be resolved. Considerations for subsequent vaccination include myocarditis or pericarditis considered unrelated to mRNA COVID-19 vaccination (e.g., due to SARS-CoV-2 or other viruses, diagnosis was made more than 3 weeks after vaccination), personal risk of severe acute COVID-19 (e.g., age, underlying conditions), or timing of any immunomodulatory therapies. Patients with a history of myocarditis or pericarditis prior to COVID-19 vaccination or more than 3 weeks after a COVID-19 vaccine dose should not receive a mRNA vaccine dose until their episode of myocarditis or pericarditis has completely resolved, which includes no evidence of ongoing heart inflammation or sequelae. The observed risk is highest in males 12 to 24 years. Based on claims data after the administration of the 2023-2024 formulation of mRNA COVID-19 vaccines, there were approximately 8 cases per million in individuals 6 months to 64 years and approximately 27 cases per million doses in males 12 to 24 years. The onset of myocarditis and pericarditis is usually within 7 days after vaccination, occurring more often after the second dose. However, cases have also been observed in females and after other doses. Although some individuals with myocarditis and/or pericarditis after administration of mRNA COVID-19 vaccines have required intensive care support, available data suggest that individuals typically have resolution of symptoms within a few days with conservative management. Persistence of abnormal cardiac magnetic resonance imaging (CMR) findings, which is a marker for myocardial injury, has been found in individuals with COVID-19 vaccine-associated myocarditis. The clinical and prognostic significance of these CMR findings are not known. Individuals who have a history of other heart disease, including congential heart disease and Kawasaki disease, may receive any currently FDA-approved or FDA-authorized COVID-19 vaccine.[66175] [66698] [66770] [67339] [67786] [72298]
COVID-19 vaccination during pregnancy is strongly recommended by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine. It is recommended as soon as possible, regardless of trimester, to optimize maternal and fetal health. There is no preferential recommendation for the use of any one COVID-19 vaccine over another.[66179] [72307] Currently, the CDC does not have the COVID-19 vaccine listed on its Adult Immunization Schedule for pregnant individuals.[72295] Analysis of current data from the V-SAFE pregnancy registry did not find an increased risk of miscarriage among approximately 2,500 pregnant individuals who received an mRNA COVID-19 vaccine before 20 weeks of pregnancy. Miscarriage rates in this population were 13%, compared to 11% to 16% of pregnancies in the general population.[72308] A systematic review and meta-analysis that included 71 clinical and preclinical studies involving pregnant individuals (n = 17,719,495) found no evidence of safety concerns associated with COVID-19 vaccination during pregnancy, with adverse outcomes not exceeding background rates. Most studies involved mRNA vaccines and showed no increased risk of adverse pregnancy outcomes.[72309] In a prospective cohort study of 131 mRNA COVID-19 vaccine recipients (84 pregnant, 31 lactating, and 16 non-pregnant), vaccine-induced antibody titers were equivalent in pregnant and lactating individuals compared to non-pregnant individuals. All titers were significantly higher than those induced during SARS-CoV-2 infection during pregnancy. Vaccine-generated antibodies were present in all umbilical cord blood samples; neutralizing antibody titers were lower in umbilical cord compared to maternal sera, although statistical significance was not reached. No differences in reactogenicity were noted between the groups.[66558] COVID-19 mRNA vaccines are not associated with infertility and safety during pregnancy has been well established. ACOG recommends vaccination for all eligible individuals who may consider future pregnancy.[66179]
COVID-19 vaccination is recommended in all eligible individuals, including those who are breast-feeding.[66179] [66864] There are limited data regarding use of the COVID-19 vaccine during breast-feeding, its effect on milk production, and its excretion in human milk.[67339] However, the COVID-19 vaccines cannot cause infection in either the lactating patient or the exposed child.[66175] Recent reports suggest lactating people who have received mRNA COVID-19 vaccines have antibodies in their milk, which may help protect neonates and infants.[66864] In a prospective cohort study of 131 mRNA COVID-19 vaccine recipients (84 pregnant, 31 lactating, and 16 non-pregnant), vaccine-induced antibody titers were equivalent in pregnant and lactating individuals compared to non-pregnant people. All titers were significantly higher than those induced during SARS-CoV-2 infection during pregnancy. Vaccine-generated antibodies were present in all milk samples. No differences in reactogenicity were noted between the groups.[66558]
The Pfizer-BioNTech COVID-19 vaccine contains nucleoside-modified messenger RNA (modRNA) and the Moderna COVID-19 vaccine is made up of a synthetic messenger RNA (mRNA), both encoding the viral spike glycoprotein (S) of SARS-CoV-2. The RNA is encapsulated in lipid nanoparticles, which enables entry into host cells, expression of the S protein, and elicitation of both antibody and cellular immune responses.[67339][67786][67907][67908][72298]
Revision Date: 10/21/2025, 01:33:00 AMThe COVID-19 vaccine is administered intramuscularly. Vaccination does not ensure immunity.[67339][67786][67907][67908]
Affected cytochrome P450 isoenzymes and drug transporters: none
After Pfizer-BioNTech COVID-19 vaccine administration, SARS-CoV-2 geometric mean titers (GMTs) 50% neutralizing (NT50) were measured in different age groups 30 days after dose 2. SARS-CoV-2 GMTs (NT50) were 1,197.6 (10 mcg/dose) in patients 5 to 11 years and 1,146.5 (30 mcg/dose) in patients 16 to 25 years with a geometric mean ratio (GMR) of 1.04. There was no difference in seroresponse rates in these age groups. SARS-CoV-2 GMTs (NT50) were 1,535.2 (3 mcg/dose) in patients 2 to 4 years and 1,180 (30 mcg/dose) in patients 16 to 25 years with a GMR of 1.3. The difference in seroresponse rates was 1.2%. SARS-CoV-2 GMTs (NT50) were 1,406.5 (3 mcg/dose) in patients 6 to 23 months and 1,180 (30 mcg/dose) in patients 16 to 25 years with a GMR of 1.19. The difference in seroresponse rates was 1.2%.[67907]
After Moderna COVID-19 vaccine administration, SARS-CoV-2 geometric mean titers (GMTs) 50% neutralizing (NT50) were measured in different age groups 28 days after dose 2. SARS-CoV-2 GMTs (NT50) were 1,401.7 in patients 12 to 17 years and 1,299.9 in patients 18 to 25 years with a geometric mean ratio (GMR) of 1.1. There was a 0.2% difference in seroresponse rates in these age groups. SARS-CoV-2 GMTs (NT50) were 1,610.2 in patients 6 to 11 years and 1,299.9 in patients 18 to 25 years with a GMR of 1.2. The difference in seroresponse rates was 0.1%. SARS-CoV-2 GMTs (NT50) were 1,410 in patients 2 to 5 years and 1,390.8 in patients 18 to 25 years with a GMR of 1. The difference in seroresponse rates was -0.4%. SARS-CoV-2 GMTs (NT50) were 1,780.7 in patients 6 to 23 months and 1,390.8 in patients 18 to 25 years with a GMR of 1.3. The difference in seroresponse rates was 0.7%.[67908]
COVID-19 vaccination during pregnancy is strongly recommended by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine. It is recommended as soon as possible, regardless of trimester, to optimize maternal and fetal health. There is no preferential recommendation for the use of any one COVID-19 vaccine over another.[66179] [72307] Currently, the CDC does not have the COVID-19 vaccine listed on its Adult Immunization Schedule for pregnant individuals.[72295] Analysis of current data from the V-SAFE pregnancy registry did not find an increased risk of miscarriage among approximately 2,500 pregnant individuals who received an mRNA COVID-19 vaccine before 20 weeks of pregnancy. Miscarriage rates in this population were 13%, compared to 11% to 16% of pregnancies in the general population.[72308] A systematic review and meta-analysis that included 71 clinical and preclinical studies involving pregnant individuals (n = 17,719,495) found no evidence of safety concerns associated with COVID-19 vaccination during pregnancy, with adverse outcomes not exceeding background rates. Most studies involved mRNA vaccines and showed no increased risk of adverse pregnancy outcomes.[72309] In a prospective cohort study of 131 mRNA COVID-19 vaccine recipients (84 pregnant, 31 lactating, and 16 non-pregnant), vaccine-induced antibody titers were equivalent in pregnant and lactating individuals compared to non-pregnant individuals. All titers were significantly higher than those induced during SARS-CoV-2 infection during pregnancy. Vaccine-generated antibodies were present in all umbilical cord blood samples; neutralizing antibody titers were lower in umbilical cord compared to maternal sera, although statistical significance was not reached. No differences in reactogenicity were noted between the groups.[66558] COVID-19 mRNA vaccines are not associated with infertility and safety during pregnancy has been well established. ACOG recommends vaccination for all eligible individuals who may consider future pregnancy.[66179]
COVID-19 vaccination is recommended in all eligible individuals, including those who are breast-feeding.[66179] [66864] There are limited data regarding use of the COVID-19 vaccine during breast-feeding, its effect on milk production, and its excretion in human milk.[67339] However, the COVID-19 vaccines cannot cause infection in either the lactating patient or the exposed child.[66175] Recent reports suggest lactating people who have received mRNA COVID-19 vaccines have antibodies in their milk, which may help protect neonates and infants.[66864] In a prospective cohort study of 131 mRNA COVID-19 vaccine recipients (84 pregnant, 31 lactating, and 16 non-pregnant), vaccine-induced antibody titers were equivalent in pregnant and lactating individuals compared to non-pregnant people. All titers were significantly higher than those induced during SARS-CoV-2 infection during pregnancy. Vaccine-generated antibodies were present in all milk samples. No differences in reactogenicity were noted between the groups.[66558]
Cookies are used by this site. To decline or learn more, visit our cookie notice.
Copyright © 2025 Elsevier, its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.