
Treatment of suspected or laboratory confirmed COVID-19 in adults and children hospitalized with severe disease (1)
India Ministry of Health and Family Welfare recommends use in moderate to severe COVID-19 disease requiring supplemental oxygen
Not to be used in a home setting or in patient not requiring supplemental oxygen (2)(3)
Initiate treatment within 10 days of symptom onset (2)
200 mg IV on day 1 followed by 100 mg IV daily for the next 4 days
40 kg or more: 200 mg IV on day 1 followed by 100 mg IV daily for the next 4 days
3.5 to 39 kg: 5 mg/kg IV on day 1 followed by 2.5 mg/kg IV daily for the next 4 days
Total treatment duration is 5 days; extending beyond 5 days is not recommended
Add 19 mL of Sterile Water for Injection
Immediately shake vial for 30 seconds; then allow to settle for 2 to 3 minutes
If not completely dissolved, repeat the shaking process until a clear solution is obtained
After reconstitution, each vial contains 100 mg/20 mL (5 mg/mL) of remdesivir solution
Total storage time after reconstitution should not exceed 4 hours at room temperature or 24 hours under refrigeration of 2 to 8 degrees C (36 to 46 degrees F)
Adults and Pediatric patients 40 kg or more:
Withdraw and discard 20 mL (100 mg dose) or 40 mL (200 mg dose) of 0.9% Sodium
Chloride from a 100 mL or 250 mL infusion bag
Transfer appropriate volume of reconstituted solution (20 or 40 mL) to the bag
Gently invert bag 20 times to mix; DO NOT shake
Diluted solution is stable for 4 hours at room temperature of 20 to 25 degrees C (68 to 77 degrees F) or 24 hours under refrigeration of 2 to 8 degrees C (35 to 46 degrees F)
Pediatric patients 3.5 to 39 kg
Follow the instructions in the tables below to prepare loading and maintenance doses using either a 25 mL, 50 mL, 100 mL, or 250 mL 0.9% Sodium Chloride infusion bag
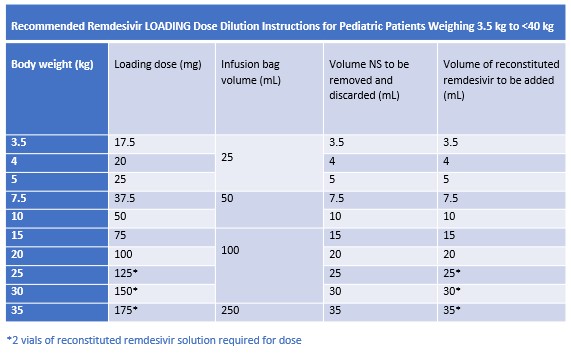
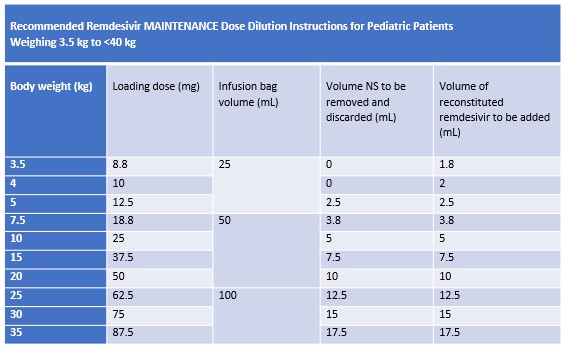
Gently invert bag 20 times to mix; DO NOT shake
Diluted solution is stable for 4 hours at room temperature of 20 to 25 degrees C (68 to 77 degrees F) or 24 hours under refrigeration of 2 to 8 degrees C (35 to 46 degrees F)
Administration:
Infuse intravenously over 30 to 120 minutes
Renal dysfunction:
o Before starting treatment, obtain creatinine clearance in all patients 29 days and older and obtain serum creatinine in neonates (7 to 28 days old) o Not recommended for use in patients 29 days and older with eGFR < 30 mL/min/m2 or in full-term neonates with serum creatinine of 1 mg/dL or higher unless potential benefits outweigh potential risks
Hepatic dysfunction:
o Perform liver function testing in all patients before starting treatment and daily during treatment o Not recommended for use in patients with ALT at least 5-times ULN at baseline o Discontinue treatment in patients who develop either of the following: o ALT at least 5-times ULN; may restart when ALT is < 5-times ULN o Elevated ALT with signs of liver inflammation or increasing conjugated bilirubin, alkaline phosphatase, or INR
Infusion-related reactions:
o Monitor for hypersensitivity and infusion-related reactions (e.g., hypotension, nausea, vomiting, diaphoresis, and shivering) o If present, immediately discontinue the infusion and initiate appropriate treatment
Pregnancy:
o Treatment of pregnant women with remdesivir has not been studied o Use during pregnancy only if potential benefits justify potential risk
Hypersensitivity and infusion-related reactions: hypotension, hypertension, sinus tachycardia, bradycardia, hypoxia, fever, dyspnea, wheezing, angioedema, rash, nausea, diaphoresis, shivering
Hepatic: elevated hepatic enzymes, hyperbilirubinemia
Renal: decreased creatinine clearance, increased creatinine, acute kidney injury or renal failure
Hematologic: decreased hemoglobin or anemia, decreased lymphocytes or lymphopenia, decreased prothrombin time
Metabolic: increased blood glucose or hyperglycemia
Neurologic: generalized seizures
Avoid concurrent administration with chloroquine or hydroxychloroquine due to antagonistic effects
References
1. Desrem (remdesivir for injection) prescribing information. Bangalore India: Mylan Laboratories Limited; 2020.
2. Directorate General of Health Services, MoHFW,GOI. Comprehensive Guidelines for Management of COVID-19 patients. Retrieved June 7, 2021. Available on the World Wide Web at: https://dghs.gov.in/WriteReadData/News/202105270436027770348ComprehensiveGuidelinesforManagementofCOVID-1927May2021DteGHS.pdf
Cookies are used by this site. To decline or learn more, visit our cookie notice.
Copyright © 2025 Elsevier, its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.