ThisiscontentfromElsevier'sDrugInformation
Intranasal Influenza Vaccine
Learn more about Elsevier’s Drug Information today! Get the reliable drug data and decision support you need to enhance patient safety through timely and accessible information.
General Dosing Information
0.2 mL/dose intranasally (0.1 mL into each nostril, for a total of 0.2 mL).[48603]
0.2 mL/dose intranasally (0.1 mL into each nostril, for a total of 0.2 mL).[48603]
0.2 mL/dose intranasally (0.1 mL into each nostril, for a total of 0.2 mL).[48603] Repeat the dose at least 4 weeks later for those who are receiving the flu vaccine for the first time. The Advisory Committee on Immunization Practices (ACIP) recommends that infants and children aged 6 months through 8 years who have previously received at least 2 doses of influenza vaccine before the beginning of the influenza season, receive only 1 dose for the current season. The 2 previous doses need not have been given during the same season or consecutive seasons.[63436]
50 years or more: Safety and efficacy have not been established.
Less than 50 years: 0.2 mL/dose intranasally.
Safety and efficacy have not been established.
0.2 mL/dose intranasally.
2 to 12 years: 0.2 mL/dose intranasally.
Less than 2 years: Safety and efficacy have not been established.
Safety and efficacy have not been established.
Safety and efficacy have not been established.
Specific guidelines for dosage adjustments in hepatic impairment are not available; it appears that no dosage adjustments are needed.
Specific guidelines for dosage adjustments in renal impairment are not available; it appears that no dosage adjustments are needed.
† Off-label indication
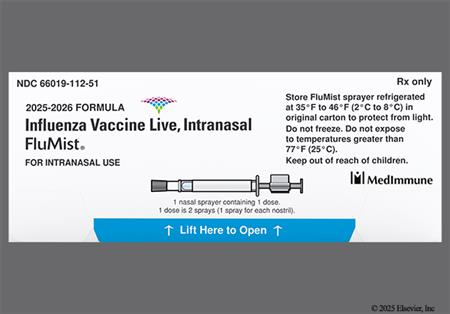
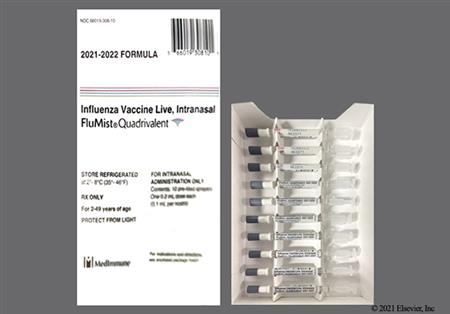

NOTE: The 2025/2026 trivalent vaccine virus strains for egg-based vaccines are an A/Victoria/4897/2022 (H1N1) pdm09-like virus, an A/Croatia/10136RV/2023 (H3N2)-like virus, and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.[48603][72015]
Intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is a liquid, cold-adapted vaccine containing live-attenuated influenza viruses that are administered as a nasal spray. Intranasal administration stimulates localized mucosal antibody formation, and because the vaccine contains attenuated viruses, an adjuvant to enhance antigen immunogenicity is not needed.[48603] The Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics (AAP) recommends annual flu vaccination with an age-appropriate influenza vaccine (i.e., inactivated influenza vaccine (IIV), recombinant influenza vaccine (RIV), or LAIV), without preference of 1 product or formulation over another, for everyone 6 months and older.[63327][63436][63520] The LAIV may not be appropriate for patients with immunosuppression. The IIV is preferred over the LAIV for vaccinating household members, health-care workers, and others who have close contact with severely immunosuppressed persons during periods when such persons require care in a protected environment. The duration of influenza vaccine virus replication and shedding after LAIV administration is unknown. As a precautionary measure, recipients of LAIV should avoid close contact with severely immunosuppressed persons for 7 days after vaccination.[63436][63520]
Updates for coronavirus disease 2019 (COVID-19):
The influenza season will coincide with the continued or recurrent circulation of SARS-CoV-2. Influenza vaccination of patients 6 months and older may reduce symptoms that might be confused with those of COVID-19. Additionally, prevention and reduction of influenza severity could decrease hospitalizations and intensive care unit admissions and alleviate stress on the health care system.[63436]
For storage information, see the specific product information within the How Supplied section.
Intranasal Administration
Health care administration
Self or caregiver administration
Adverse reactions reported in adults and children receiving the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) include nasal congestion (9% to 58%), rhinorrhea (44% to 58%), sneezing (2%), sore throat (5% to 28%), irritability (12% to 21%), headache (3% to 40%), lethargy (7% to 14%), weakness (26%), sinusitis (4%), fever of more than 100 degrees F (13% to 16%), myalgia (2% to 17%), anorexia (13% to 21%), abdominal pain (2% to 12%), chills (2% to 9%), and cough (14%). More patients who received the vaccine than those who received placebo had otitis media (3% vs. 1%, respectively).[48603]
No cases of Guillain-Barre syndrome (GBS) have been reported with the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)). However, an increased risk of developing GBS after administration of the inactivated influenza virus vaccine was evident in 1976. Subsequent influenza vaccinations during the next few years were not associated with this increased risk. However, during 1993 to 94 twice the average yearly rate (for the period 1990 to 95) of influenza vaccine-associated incidences of GBS were reported, representing a risk of slightly more than 1 additional case of GBS per million persons vaccinated. In most cases, symptoms were limited and reversible, but fatalities have occurred. Persons with a history of GBS have a substantially greater likelihood of subsequently developing GBS than persons without such a history. Thus, the likelihood of coincidentally developing GBS after influenza vaccination is expected to be greater among persons with a history of GBS than among persons with no history of this syndrome. It is not known if influenza vaccination is causally associated with this risk for recurrence of GBS.[48603] [52656]
While wheezing can occur in any patient after intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) receipt, children younger than 5 years of age with a history of recurrent wheezing and patients with asthma may be at higher risk for wheezing after vaccine administration compared to other age groups. In 1 clinical trial comparing intranasal influenza vaccine to an inactivated influenza virus vaccine (by Sanofi Pasteur Inc.), 2.1% (47/2,187) of children 24 to 59 months experienced wheezing in the intranasal influenza vaccine group compared to 2.5% (56/2,198) in the active control group. In clinical trials, infants and children 6 to 23 months old had a higher risk for wheezing (5.9%) and hospitalization (4.2%); therefore, intranasal influenza vaccine is not FDA-approved for use in this population.[48603]
Rarely, immediate allergic reactions occur after influenza vaccination in all populations. An allergic reaction may present as urticaria, rash, angioedema, bronchospasm, and/or anaphylactic shock. Hypersensitivity reactions, including anaphylactoid reactions, facial edema, and urticaria, have been reported during postmarketing surveillance in people receiving the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)).[48603]
Nausea, vomiting, diarrhea, pericarditis, syncope, meningitis, eosinophilic meningitis, exacerbation of symptoms of mitochondrial encephalomyopathy (Leigh syndrome), Bell's palsy, and vaccine-associated encephalitis have been reported during postmarketing surveillance in people receiving the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)).[48603]
The coadministration of certain medications may lead to harm and require avoidance or therapy modification; review all drug interactions prior to concomitant use of other medications.
This medication is contraindicated in patients with a history of hypersensitivity to it or any of its components. The FDA-approved product labeling states that intranasal influenza vaccine (LAIV) is also contraindicated in individuals with egg hypersensitivity because these vaccines are prepared using embryonated chicken eggs.[48603] The Advisory Committee on Immunization Practices (ACIP) recommends administering any licensed, age-appropriate influenza vaccine (i.e., inactivated influenza vaccine (IIV), recombinant influenza vaccine (RIV), or LAIV) to patients with egg allergy of any severity. Individuals who have had symptoms other than urticaria after egg exposure (i.e., angioedema, respiratory distress, lightheadedness, or recurrent emesis) or required emergency medical intervention, may also receive any licensed, age-appropriate influenza vaccine; however, it is recommended that the vaccine only be administered in an inpatient or outpatient medical setting by a health care provider experienced in the recognition and management of severe allergic conditions. Although no specific observation time is recommended for egg-allergic patients, ACIP recommends patients be observed for syncope for 15 minutes after vaccination.[53026][63436] The American Academy of Pediatrics (AAP), the American Academy of Allergy, Asthma, and Immunology (AAAAI), and the American College of Allergy, Asthma, and Immunology (ACAAI) do not recommend any special precautions for egg-allergic recipients, regardless of severity. The AAAAI and ACAAI consider any special precautions (i.e., special observation periods or administration in a specialized medical setting) unnecessary due to the extremely rare risk of anaphylactic reactions after vaccination. They recommend that providers and screening questionnaires not ask about the egg allergy status of the influenza virus vaccine recipient.[62809][63520]
Deferral of intranasal influenza vaccine administration may be appropriate for a patient with a moderate or severe acute illness such as infection with or without fever. The Advisory Committee on Immunization Practices recommends that vaccinations be delayed during the course of a moderate or severe acute febrile illness and administered after the acute phase of illness has resolved. All vaccines can be administered to persons with minor illnesses such as diarrhea, mild upper-respiratory infection with or without low-grade fever, or other low-grade febrile illness. Vaccinate persons with moderate or severe febrile illness as soon as they have recovered from the acute phase of the illness. However, if nasal congestion is present that might impede delivery of the vaccine to the nasopharyngeal mucosa, then defer administration of the intranasal vaccine until the congestion has resolved or administer the inactivated influenza vaccine.[63520][65107] Allergic rhinitis does not preclude intranasal influenza vaccine administration. There are no data regarding concurrent intranasal corticosteroid administration and the use of intranasal influenza vaccine.[52656]
The effectiveness of intranasal influenza vaccine has not been evaluated in individuals with significant immunosuppression.[43236] Immunosuppressed persons may include patients with asymptomatic or symptomatic human immunodeficiency virus (HIV) infection; severe combined immunodeficiency (SCID); hypogammaglobulinemia; agammaglobulinemia; altered immune states due to diseases such as leukemia, lymphoma, or generalized neoplastic disease; or an immune system compromised by corticosteroid therapy with greater than physiologic doses, alkylating drugs, antimetabolites, or radiation. Short-term (less than 2 weeks) corticosteroid therapy or intra-articular, bursal, or tendon injections with corticosteroids should not be immunosuppressive.[31601] The intranasal influenza vaccine is not recommended for close contacts of severely immunosuppressed persons who require a protected environment. Individuals with leukemia, lymphoma, or other malignancies whose disease is in remission and whose chemotherapy or radiation therapy has been terminated for at least 3 months can receive live-virus vaccines.[43236] [53026] The vaccine should be avoided in patients with anatomical or functional asplenia, anyone with cranial cerebrospinal fluid leak, or cochlear implant. Patients with cochlear implants are at risk for cerebrospinal (CSF) leak for a period after implantation.[43236] [53026] [63436] [63520] The efficacy of the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) has not been established in patients with HIV infection or acquired immunodeficiency syndrome (AIDS). The guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV considers use of the live attenuated influenza vaccine (LAIV) administered via the nasal route as contraindicated in patients with HIV.[34362] The live virus vaccine theoretically could cause serious influenza infection in these patients. However, among 28 adult patients with HIV infection (median CD4 count of 541 cells/mm3), no serious adverse reactions were reported during the 4 weeks after vaccine receipt. Vaccine strain (type B) virus was detected in 1 of 28 HIV-infected subjects on Day 5 only.[48603] The CDC recommends immunization of HIV-infected individuals with the inactivated influenza vaccine, as this measure is safe and may confer protection against influenza.[63436] [63520]
The intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is not the preferred agent for use in patients with a history of asthma or reactive airway disease, particularly pediatric patients younger than 5 years old with recurrent wheezing or bronchospasm, due to the increased risk of wheezing after administration; weigh the potential benefit against the potential risk. In a placebo-controlled safety study, an increase in asthma events was observed among patients younger than 5 years of age. Do not administer LAIV to patients with severe asthma or active wheezing because these individuals have not been studied in clinical trials.[48603] The inactivated influenza vaccine is the recommended product for patients with asthma or a history of wheezing in the past 12 months (for pediatric patients ages 2 to younger than 5 years).[63436]
The intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is not indicated for use in geriatric patients; the vaccine is only indicated for patients 2 to 49 years of age. The effectiveness of the vaccine was not demonstrated in a clinical trial with a subgroup of patients 50 to 64 years of age.[48603]
Neonates, infants, and children younger than 2 years of age should not receive the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)). Instead, administer the inactivated influenza vaccine to infants and children 6 months to 2 years of age. In clinical trials, an increased risk of wheezing that required bronchodilator therapy or was associated with significant respiratory symptoms within 6 weeks of vaccination, and hospitalization (for any cause within 6 months) was observed among pediatric patients younger than 2 years who received LAIV (5.9% and 4.2%, respectively) compared with those who received inactivated influenza vaccine (3.8% and 3.2%, respectively).[48603]
The intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is not absorbed systemically after intranasal administration and use during pregnancy is not expected to result in fetal exposure to the drug.[48603] However, according to the Advisory Committee on Immunization Practices (ACIP), administration of live vaccines to pregnant individuals should be avoided. Because pregnancy may increase the risk of serious medical complications from influenza, the ACIP recommends that all people who are pregnant or will be pregnant (in any trimester) during influenza season be routinely vaccinated with an inactivated influenza virus vaccine.[63436]
The safety of the intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) in individuals with underlying medical conditions that may predispose them to complications after wild-type influenza infection (e.g., chronic lung disease, cardiovascular disease, renal disease, metabolic disease (including diabetes mellitus), neurological disease, hematological disease, or hepatic disease) has not been established; only administer the intranasal influenza vaccine if the potential benefit outweighs the potential risk.[48603] [63436] The American Academy of Pediatrics specifically recommends the use of the inactivated influenza virus vaccine for all pediatric patients with the following conditions: asthma; chronic pulmonary diseases, including chronic lung disease (CLD) and cystic fibrosis; hemodynamically significant cardiac disease; immunosuppressive disorders, including HIV infection; sickle cell disease or other hemoglobinopathy; chronic renal dysfunction (e.g., renal failure); chronic metabolic disease (e.g., diabetes mellitus); conditions that require long-term aspirin therapy, such as juvenile idiopathic arthritis or Kawasaki disease; and any condition that can compromise respiratory function or handling of secretions (e.g., neuromuscular disease such as multiple sclerosis, seizure disorder, spinal cord injuries, neurodevelopmental disorders).[63520]
Intranasal influenza vaccine is compatible with breast-feeding. Intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is not absorbed systemically after intranasal administration; hence, breast-feeding is not expected to result in exposure of the child to the intranasal influenza vaccine.[48603] According to the Advisory Committee on Immunization Practices (ACIP), live attenuated influenza vaccine is safe for use in breast-feeding individuals. Although live viruses in vaccines can replicate in vaccine recipients, the majority of live viruses in vaccines are not excreted in human milk. If infection does occur, it is well tolerated because the virus is attenuated. There are no data to suggest that passive transfer of antibodies in human milk affects the efficacy of live-virus vaccines. Breastfed children should be vaccinated according to the recommended schedule.[43236]
The intranasal influenza vaccine should be used with caution in individuals who have experienced a history of significant adverse event(s) associated with previous use. If Guillain-Barre syndrome (GBS) occurs within 6 weeks of receipt of a prior influenza vaccine, the decision to give the intranasal influenza vaccine should be based on careful consideration of the potential benefits and possible risks.[48603] Persons with a history of GBS have a substantially greater likelihood of subsequently developing GBS than persons without such a history. It is not known whether influenza vaccination is causally associated with this risk for recurrence of GBS. Nevertheless, as a precaution, the ACIP generally recommends against influenza immunization in patients who have developed GBS within 6 weeks of a previous influenza vaccination. However, for most persons with a history of GBS who are at high risk for severe complications from influenza, many experts believe the benefits of the influenza virus vaccine justify yearly vaccination.[63436]
The immune mechanisms that confer protection from influenza infection have not been fully elucidated. Intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) imparts immunity against the influenza virus by stimulating production of antibodies that are specific to the disease. Influenza strain-specific serum antibodies to the vaccine have been demonstrated. The intranasal route of administration also stimulates localized mucosal antibody formation and may enhance cytotoxic T-cell formation. In general, patients who receive the vaccine will be immune only to those strains of the virus from which the vaccine was prepared. In one clinical study, protection was afforded against an H3N2 strain that was not well-matched to the H3N2 strain contained in the virus, suggesting that the vaccine provided cross-protection against the variant strain.[27509][48603]
Vaccine recipients develop immunity only to those strains of the virus from which the vaccine was prepared. Influenza viruses are recognized by the surface antigens they carry, and two such antigens, hemagglutinin (H) and neuraminidase (N) have been identified and are used to classify the various viruses. Subtypes of these strains (H1, H2, H3, N1, N2) are associated with influenza A virus and have been recognized to cause disease in humans. Immunity to these surface antigens increases resistance to infection and decreases the severity of the disease if infection occurs. Eventually, antigenic variation can occur, and immunity to one strain may no longer impart immunity to distantly related subtypes of the virus. Influenza B viruses also exhibit antigenic variation, and new variants of both types of viruses continue to cause widespread epidemics of respiratory disease.[63436]
The intranasal influenza vaccine is attenuated and thus, will not produce classic influenza-like illness. However, as the product is a live vaccine, some flu-like symptoms may occur shortly after vaccine receipt. The frequency and duration of viral shedding after vaccine receipt have not been established. At least one vaccine strain was recovered from 80% of intranasal influenza vaccine recipients during the monitoring period, which was the first 21 days after vaccine receipt. The mean duration of vaccine strain detection was 7.6 +/- 3.4 days. In a transmission study in a daycare setting (197 infants and children 8 to 36 months of age), the transmission rate was estimated to be 0.58% (95% CI, 0 to 1.7) to 2.4% (95% CI, 0.13 to 4.6%). In viral shedding studies, the large majority of patients who shed at least 1 detectable virus strain did so before day 11 post vaccination as follows: 6 to 23 months of age: 89% shed at least 1 detectable virus strain within 28 days after vaccination, 7% had detectable shedding after day 11; 24 to 59 months of age: 69% shed at least 1 detectable virus strain within 28 days after vaccination, 1% had detectable shedding after day 11; 5 to 8 years of age: 50% shed at least 1 detectable virus strain within 28 days after vaccination, 2.9% had detectable shedding after day 11; 9 to 17 years of age: 29% shed at least 1 detectable virus strain within 28 days after vaccination, 1.6% had detectable shedding after day 11; adults 18 to 49 years of age: 20% shed at least 1 detectable virus strain within 28 days after vaccination, 0.9% had detectable shedding after day 11.[48603]
Revision Date: 09/10/2025, 01:32:00 AMThe intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is administered nasally as a fine mist to the inside of the nose. Most of the dose is deposited in the nose and nasopharynx (89.7% in adults); a minimal amount is distributed into the stomach (2.6%), brain (2.4%), and lung (0.4%). The clinical significance of these distribution data is unknown. The duration of immunity imparted by the intranasal influenza vaccine is unknown; annual revaccination is recommended.[52656]
The intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is not absorbed systemically after intranasal administration and use during pregnancy is not expected to result in fetal exposure to the drug.[48603] However, according to the Advisory Committee on Immunization Practices (ACIP), administration of live vaccines to pregnant individuals should be avoided. Because pregnancy may increase the risk of serious medical complications from influenza, the ACIP recommends that all people who are pregnant or will be pregnant (in any trimester) during influenza season be routinely vaccinated with an inactivated influenza virus vaccine.[63436]
Intranasal influenza vaccine is compatible with breast-feeding. Intranasal influenza vaccine (live attenuated influenza vaccine (LAIV)) is not absorbed systemically after intranasal administration; hence, breast-feeding is not expected to result in exposure of the child to the intranasal influenza vaccine.[48603] According to the Advisory Committee on Immunization Practices (ACIP), live attenuated influenza vaccine is safe for use in breast-feeding individuals. Although live viruses in vaccines can replicate in vaccine recipients, the majority of live viruses in vaccines are not excreted in human milk. If infection does occur, it is well tolerated because the virus is attenuated. There are no data to suggest that passive transfer of antibodies in human milk affects the efficacy of live-virus vaccines. Breastfed children should be vaccinated according to the recommended schedule.[43236]
Cookies are used by this site. To decline or learn more, visit our cookie notice.
Copyright © 2025 Elsevier, its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.